Hydrogen Gas And Nitrogen Gas React To Form Ammonia Gas.
Hydrogen Gas And Nitrogen Gas React To Form Ammonia Gas. - Hydrogen gas and nitrogen gas react to form ammonia gas. Web chemistry chemistry questions and answers hydrogen gas reacts with nitrogen gas to form ammonia gas write a balanced chemical equation for this reaction ローロ x ?. Nitrogen and hydrogen gases react to form ammonia gas via the following reaction: Web nitrogen and hydrogen combine to form ammonia via the following reaction: 1 n2 (s) + 3 h2 (g) → 2 nh3 (g)what mass of nitrogen is required to completely react with 800.0 ml. Web how many moles of ammonia gas are produced in the following reaction if 25 moles of nitrogen gas reacts with hydrogen? Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as; Web nitrogen gas and hydrogen gas react to form ammonia gas via the folowing reaction. Web in a chemical reaction the starting materials are treated as reactants. N2 (g) + 3h2 (g) +2nh3 (g) at a certain temperature and pressure, 1.1 l of n2.
Web hydrogen gas and nitrogen gas react to form ammonia gas. What volume of ammonia would be produced by this reaction if 8.8 m² of nitrogen were consumed? The volume of nitrogen (n₂) gas = 7.7 ml. In the reaction of nitrogen gas, n2, with hydrogen gas, h2, to form ammonia gas, nh3, how many moles of hydrogen are needed to react with two moles of. Thus, 7.7 ml = 0.0077 l. Web nitrogen and hydrogen combine to form ammonia via the following reaction: Web the balanced equation for the reaction is: 1 n2 (s) + 3 h2 (g) → 2 nh3 (g)what mass of nitrogen is required to completely react with 800.0 ml. Extra content n2(g) + 3h2(g). Hydrogen gas and nitrogen gas react to form ammonia gas.
Web nitrogen gas reacts with hydrogen gas to form ammonia gas. N2 (nitrogen) + 3h2 (hydrogen) <=> 2nh3 (ammonia),. Thus, 7.7 ml = 0.0077 l. The volume of nitrogen (n₂) gas = 7.7 ml. 1 n2 (s) + 3 h2 (g) → 2 nh3 (g)what mass of nitrogen is required to completely react with 800.0 ml. Web in a chemical reaction the starting materials are treated as reactants. In the chemical equation above, 3 moles of hydrogen gas react with 1 mole of nitrogen gas to produce 2 moles of ammonia gas. Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as; Web chemistry chemistry questions and answers hydrogen gas reacts with nitrogen gas to form ammonia gas write a balanced chemical equation for this reaction ローロ x ?. Web 1 day agofortunately, ammonia is a promising hydrogen carrier that can be easily liquified under milder conditions, transported, and decomposed with the help of a catalyst.
Solved 10 g of nitrogen is reacted with 5.0 g of hydrogen to
N₂ (g) + 3 h₂ (g) → 2 nh₃ (g) the. $$ \mathrm{n}_{2}(g)+3 \mathrm{h}_{2}(g) \longrightarrow 2 \mathrm{nh}_{3}(g) $$ at a. Web nitrogen gas reacts with hydrogen gas to form ammonia gas. Web hydrogen gas and nitrogen gas react to form ammonia gas. In the reaction of nitrogen gas, n2, with hydrogen gas, h2, to form ammonia gas, nh3, how many.
PPT Reaction Rates PowerPoint Presentation, free download ID6516140
Nitrogen and hydrogen gases react to form ammonia gas via the following reaction: Web in a chemical reaction the starting materials are treated as reactants. Web nitrogen and hydrogen combine to form ammonia via the following reaction: Web nitrogen gas reacts with hydrogen gas to form ammonia gas. N₂ (g) + 3 h₂ (g) → 2 nh₃ (g) the.
PPT Covalent Bonding PowerPoint Presentation ID5648526
Web the balanced equation for the reaction is: Web hydrogen gas and nitrogen gas react to form ammonia gas. What volume of ammonia would be produced by this reaction if 8.8 m² of nitrogen were consumed? N₂ (g) + 3 h₂ (g) → 2 nh₃ (g) the. Thus, 7.7 ml = 0.0077 l.
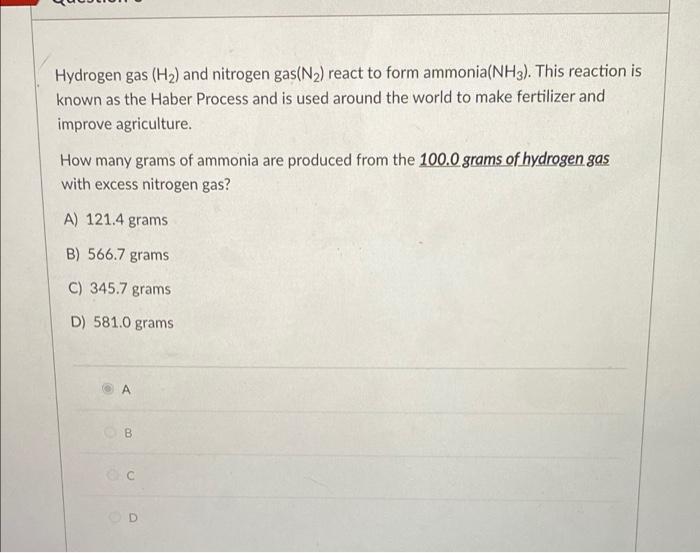
Solved Hydrogen gas (H2) and nitrogen gas(N2) react to form
Web nitrogen gas reacts with hydrogen gas to form ammonia gas. Web nitrogen and hydrogen gases react to form ammonia gas as follows: Hydrogen gas and nitrogen gas react to form ammonia gas. Web nitrogen gas and hydrogen gas react to form ammonia gas via the folowing reaction. N2 (g)+3h2 (g)↽−−⇀2nh3 (g) when 0.600 mol n2 and 0.400 mol h2.
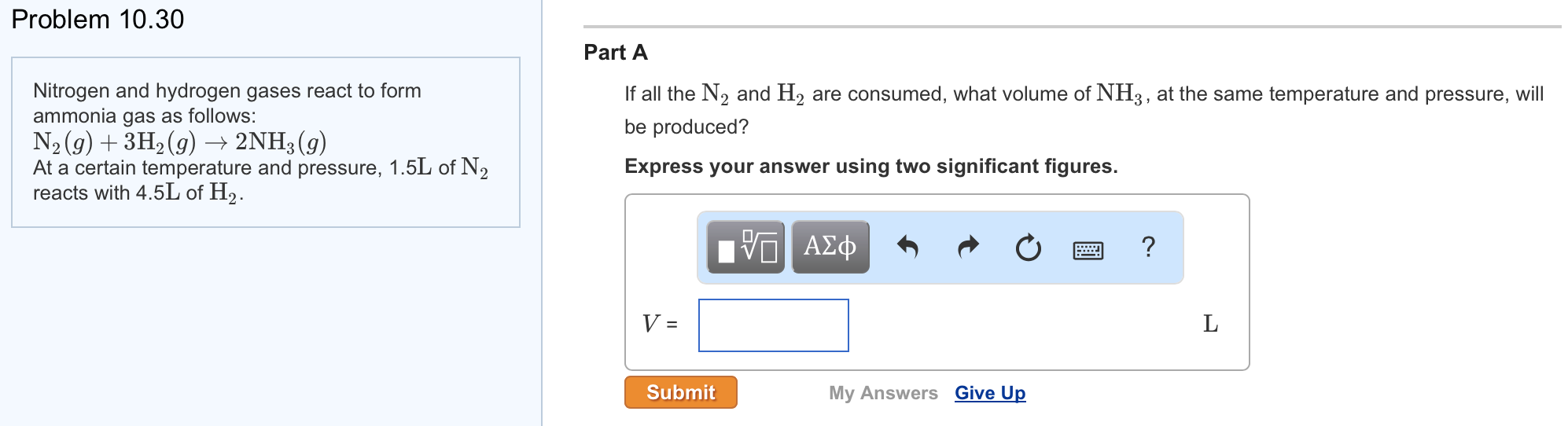
Solved Nitrogen And Hydrogen Gases React To Form Ammonia
Web how many moles of ammonia gas are produced in the following reaction if 25 moles of nitrogen gas reacts with hydrogen? In the reaction of nitrogen gas, n2, with hydrogen gas, h2, to form ammonia gas, nh3, how many moles of hydrogen are needed to react with two moles of. N2 (g)+3h2 (g)↽−−⇀2nh3 (g) when 0.600 mol n2 and.
Solved Nitrogen (N2) gas and hydrogen (H2) gas react to form
N2 (g) + 3h2 (g) +2nh3 (g) at a certain temperature and pressure, 1.1 l of n2. What volume of ammonia would be produced by this reaction if 8.8 m² of nitrogen were consumed? Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as; Web nitrogen and hydrogen gases.
Nitrogen gas and hydrogen gas combine to form gaseous ammonia
Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as; 1 ml = 0.001 l. Web the balanced equation for the reaction is: N₂ (g) + 3 h₂ (g) → 2 nh₃ (g) the. N2 (g)+3h2 (g)↽−−⇀2nh3 (g) when 0.600 mol n2 and 0.400 mol h2 are placed in.
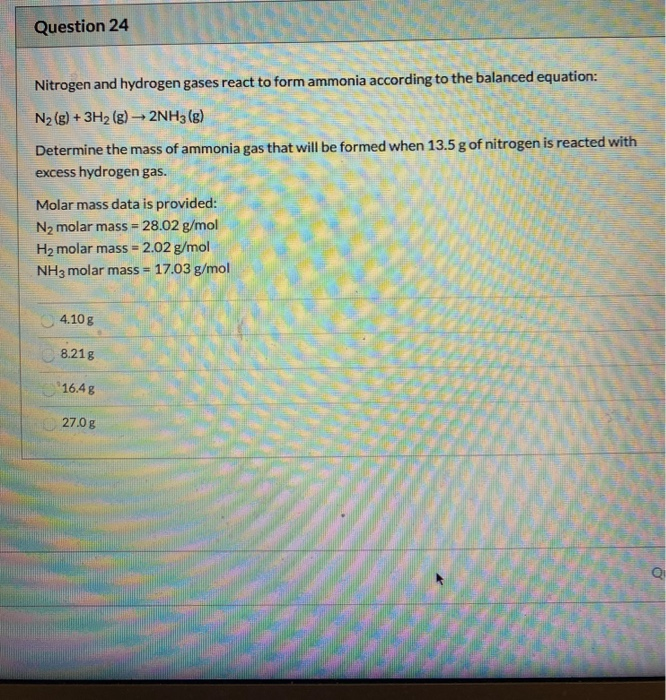
Solved Question 24 Nitrogen and hydrogen gases react to form
Web the balanced equation for the reaction is: 1 ml = 0.001 l. In the chemical equation above, 3 moles of hydrogen gas react with 1 mole of nitrogen gas to produce 2 moles of ammonia gas. Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as; N2 (g)+3h2.
[Best Answer] hydrogen gas combines with nitrogen to form Ammonia
$$ \mathrm{n}_{2}(g)+3 \mathrm{h}_{2}(g) \longrightarrow 2 \mathrm{nh}_{3}(g) $$ at a. Web hydrogen gas and nitrogen gas react to form ammonia gas. N2 (g) + 3h2 (g) +2nh3 (g) at a certain temperature and pressure, 1.1 l of n2. 1 n2 (s) + 3 h2 (g) → 2 nh3 (g)what mass of nitrogen is required to completely react with 800.0 ml. Extra.
D Question 9 1 pts Nitrogen and hydrogen gas react to form ammonia (NH3
What volume of ammonia would be produced by this reaction if 5.53 ml of hydrogen. Web the balanced equation for the reaction is: $$ \mathrm{n}_{2}(g)+3 \mathrm{h}_{2}(g) \longrightarrow 2 \mathrm{nh}_{3}(g) $$ at a. Hydrogen gas and nitrogen gas react to form ammonia gas. Web nitrogen and hydrogen gases react to form ammonia gas as follows:
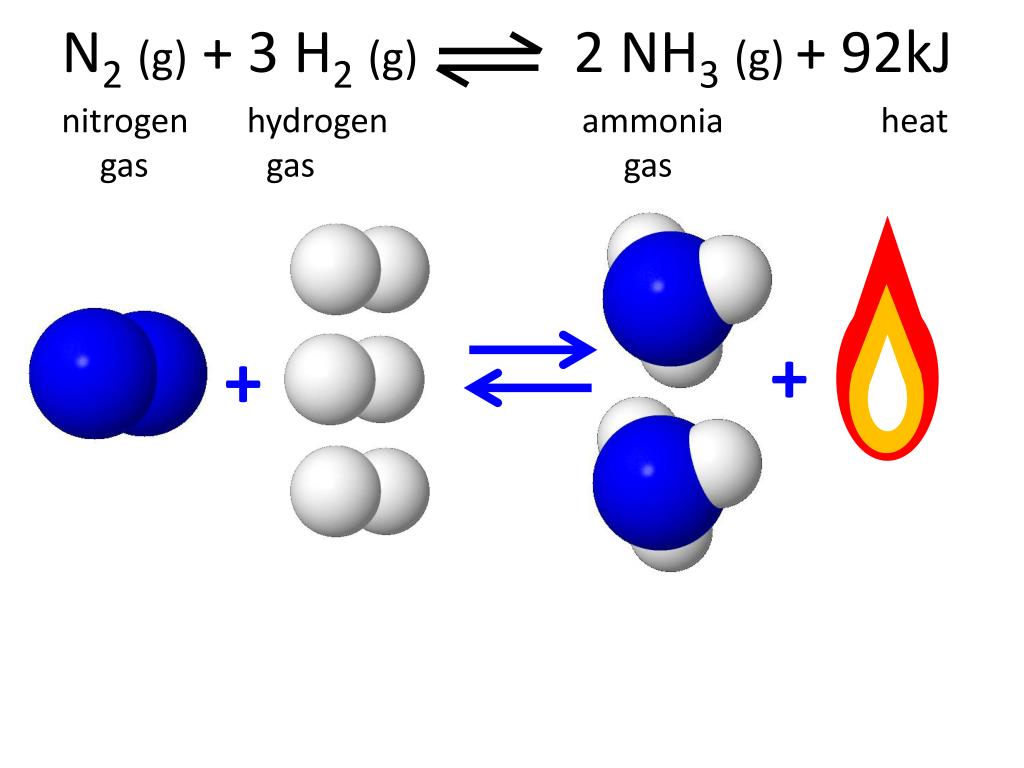
Extra Content N2(G) + 3H2(G).
Hydrogen gas and nitrogen gas react to form ammonia gas. Web nitrogen and hydrogen combine to form ammonia via the following reaction: Thus, 7.7 ml = 0.0077 l. Web how many moles of ammonia gas are produced in the following reaction if 25 moles of nitrogen gas reacts with hydrogen?
Web 1 Day Agofortunately, Ammonia Is A Promising Hydrogen Carrier That Can Be Easily Liquified Under Milder Conditions, Transported, And Decomposed With The Help Of A Catalyst.
Web in a chemical reaction the starting materials are treated as reactants. The volume of nitrogen (n₂) gas = 7.7 ml. Web the balanced equation for the reaction is: N₂ (g) + 3 h₂ (g) → 2 nh₃ (g) the.
$$ \Mathrm{N}_{2}(G)+3 \Mathrm{H}_{2}(G) \Longrightarrow 2 \Mathrm{Nh}_{3}(G) $$ At A.
Web chemistry chemistry questions and answers hydrogen gas reacts with nitrogen gas to form ammonia gas write a balanced chemical equation for this reaction ローロ x ?. H 2 ( g) hydrogen + n 2 ( g) nitrogen ⇌ nh 3 ( g). What volume of ammonia would be produced by this reaction if 8.8 m² of nitrogen were consumed? N2 (nitrogen) + 3h2 (hydrogen) <=> 2nh3 (ammonia),.
1 N2 (S) + 3 H2 (G) → 2 Nh3 (G)What Mass Of Nitrogen Is Required To Completely React With 800.0 Ml.
In the reaction of nitrogen gas, n2, with hydrogen gas, h2, to form ammonia gas, nh3, how many moles of hydrogen are needed to react with two moles of. Web hydrogen gas and nitrogen gas react to form ammonia gas. Web nitrogen gas and hydrogen gas react to form ammonia gas via the folowing reaction. Nitrogen and hydrogen gases react to form ammonia gas via the following reaction:



![[Best Answer] hydrogen gas combines with nitrogen to form Ammonia](https://hi-static.z-dn.net/files/dc1/9e1c135271e8d11c1d9621fa70f7ea35.jpg)
