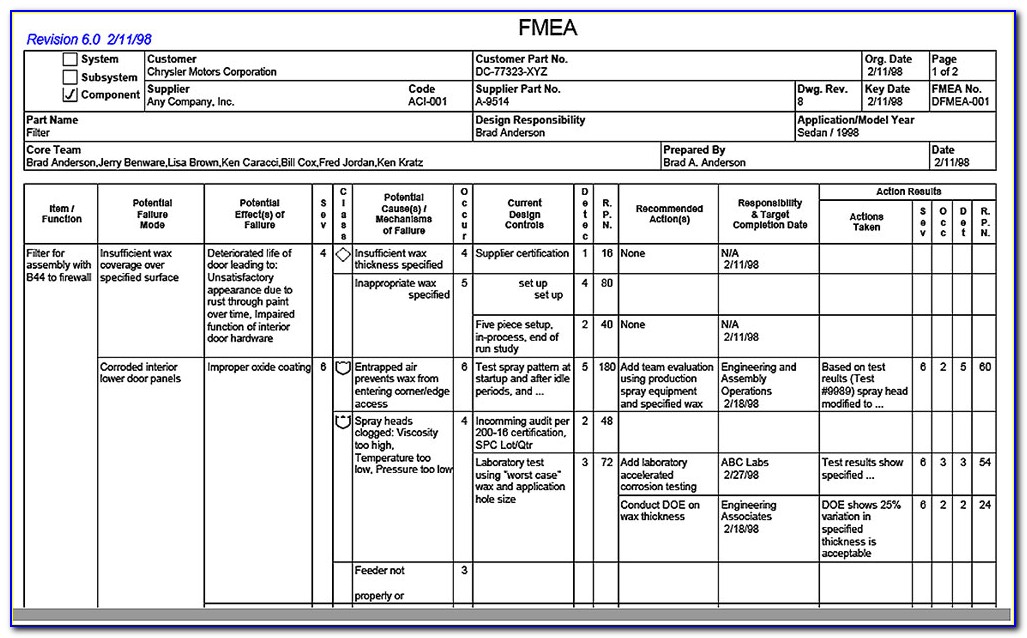
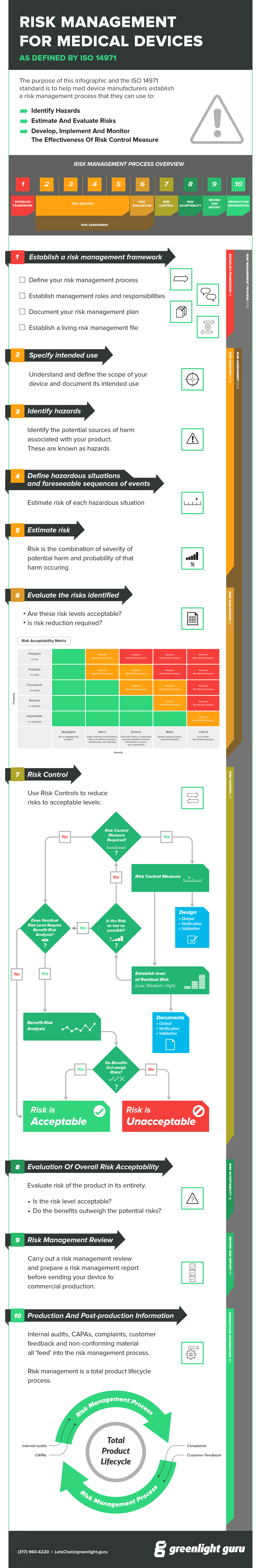
Iso 14971:2019 Risk Management Plan Template
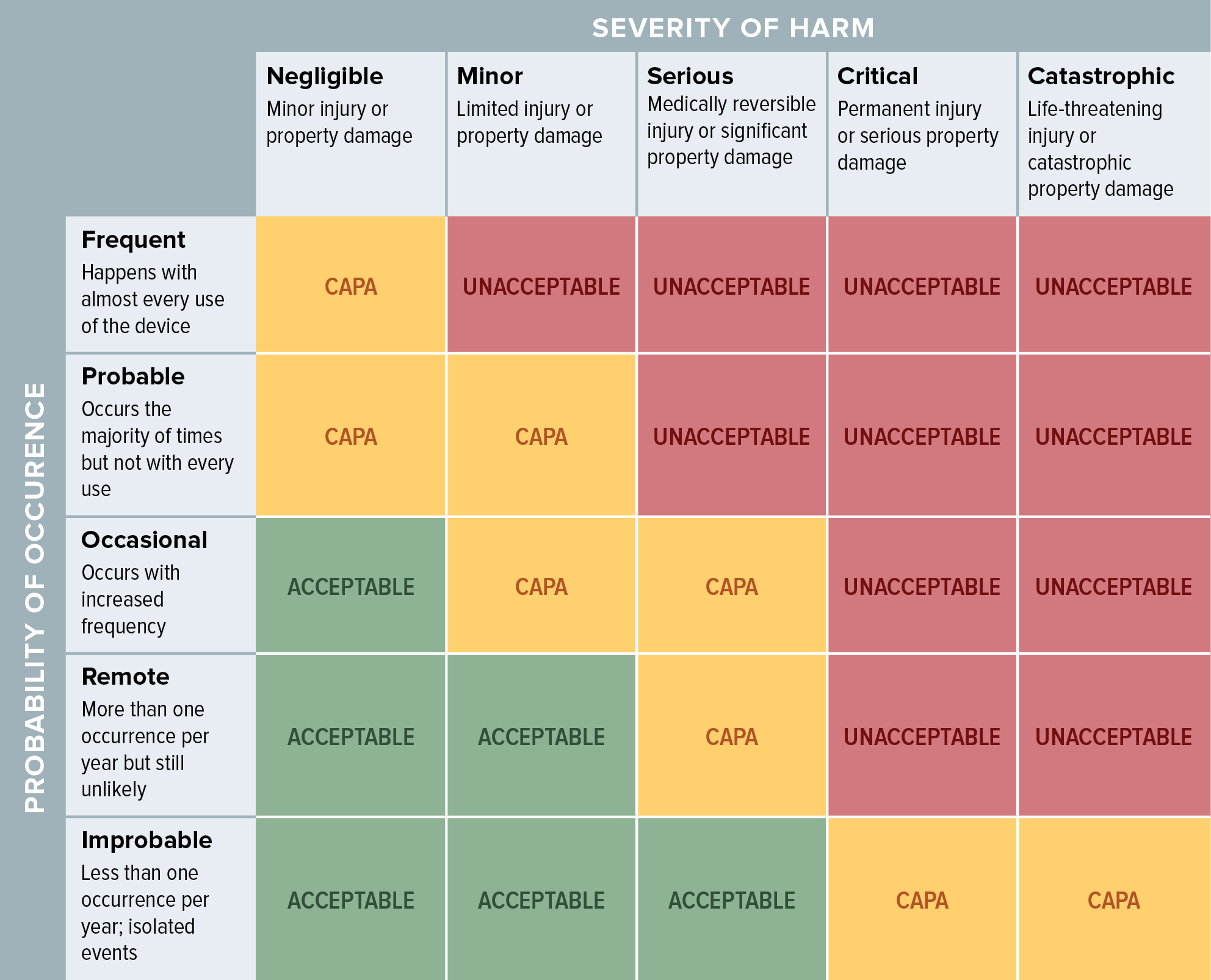
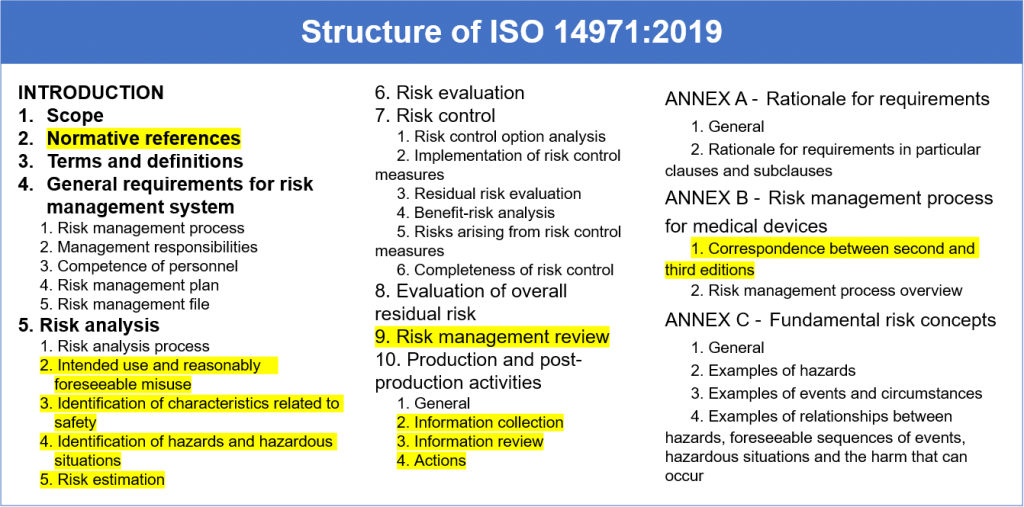
Iso 14971:2019 Risk Management Plan Template - Web iso 14971:2019 medical devices — application of risk management to medical devices abstract preview this document specifies terminology, principles and a process for risk. Web established principles of risk management that have evolved over many years. Web recently, the third version of iso 14971:2019 series has been notified and several aspects of this regulation include the best objectives to be achieved by the. Web what's new in iso 14971:2019 key definitions implementing iso 14971 initiating risk management and design controls part 1: Web templates iso 14971 templates updated june 20, 2023 template: For the particular medical device being considered, the. Web iso 14971 specifies a process through which the manufacturer of a medical device can identify hazards associated with a medical device, estimate and evaluate the risks. Click here to get this previously confidential risk management plan template in accordance with the requirements of iso 14971:2019 or. Web the method for the evaluation of the overall residual risk and the criteria for its acceptability are required to be defined in the risk management plan. Web the purpose of this procedure is to describe the risk management process in accordance with iso 14971.
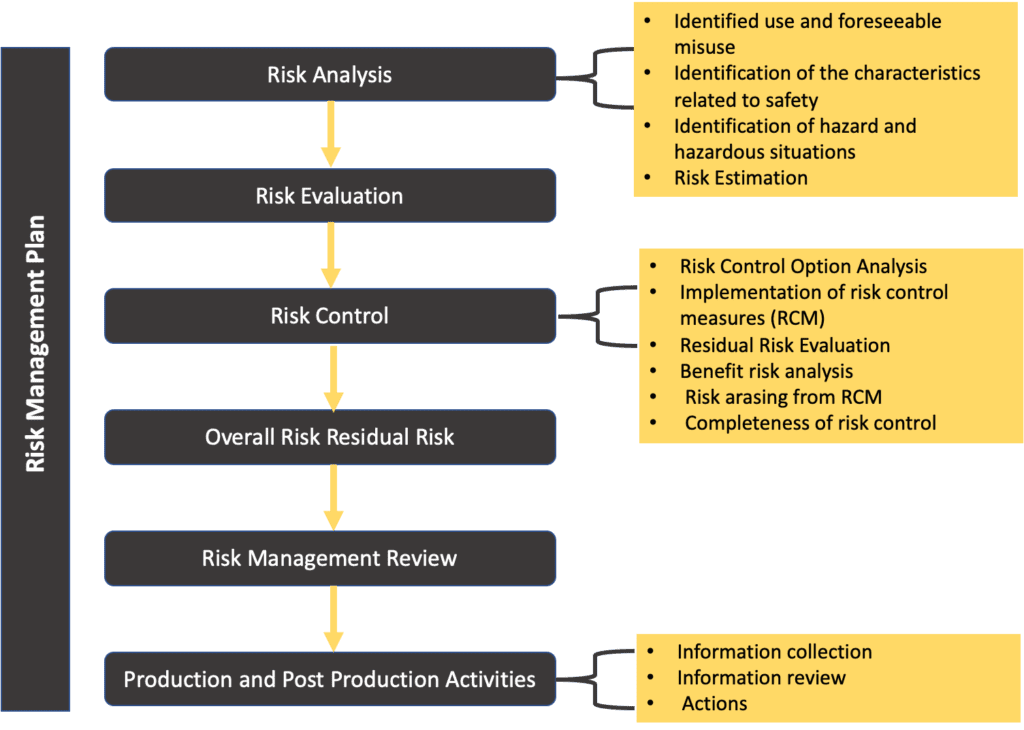
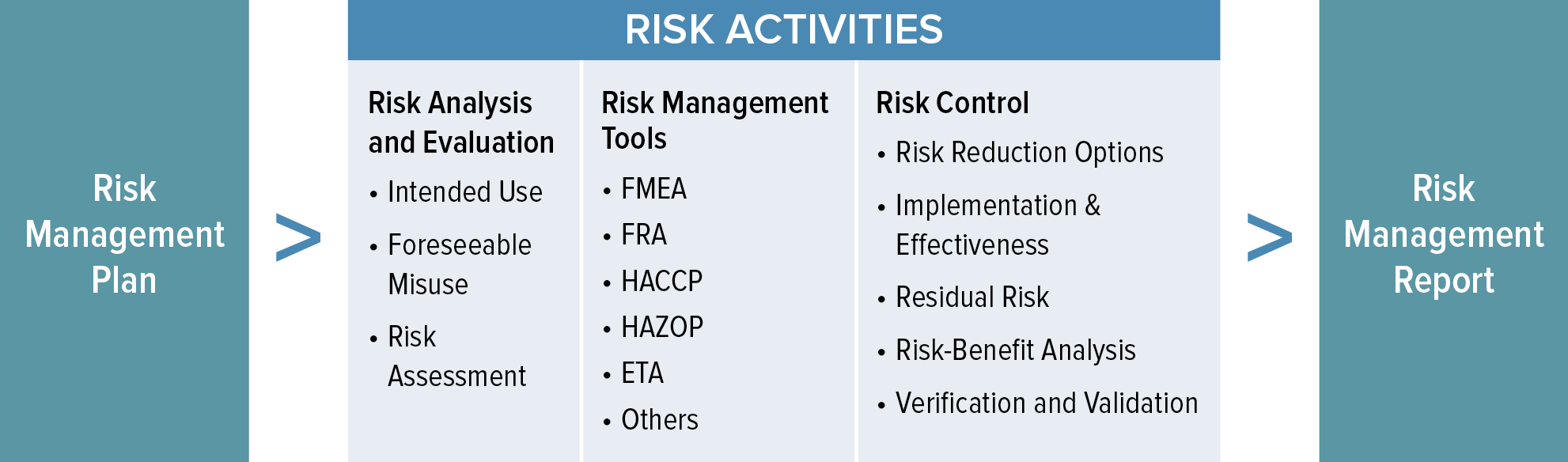
Web templates iso 14971 templates updated june 20, 2023 template: For the particular medical device being considered, the. Web the risk management process described in the new iso 14971 consists of several steps: This document could be used as guidance in developing and maintaining a risk management. Web iso 14971:2019 is the international standard for risk management in medical devices. The focus of this blog post is the first of these six steps: Iso 14971:2019 has been recognized as the consensus standard by. Web recently, the third version of iso 14971:2019 series has been notified and several aspects of this regulation include the best objectives to be achieved by the. Web the risk management plan. Web established principles of risk management that have evolved over many years.
Web iso 14971:2019 medical devices — application of risk management to medical devices abstract preview this document specifies terminology, principles and a process for risk. Web the method for the evaluation of the overall residual risk and the criteria for its acceptability are required to be defined in the risk management plan. Web established principles of risk management that have evolved over many years. For the particular medical device being considered, the. Web an iso 14971 checklist is a form based on the guidelines of iso 14971, a voluntary international standard that details how to apply risk management practices for. A new requirement to establish a method to evaluate the overall residual risk and criteria for. Web iso 14971:2019 is the international standard for risk management in medical devices. Web templates iso 14971 templates updated june 20, 2023 template: Web the risk management process described in the new iso 14971 consists of several steps: Click here to get this previously confidential risk management plan template in accordance with the requirements of iso 14971:2019 or.
ISO 14971 2019 Information & Training Medical DevicesPresentationEZE
Oliver eidel template download this is a free template,. Web templates iso 14971 templates updated june 20, 2023 template: Web in iso 14971:2019, section 4.4, the standard states that: Web what's new in iso 14971:2019 key definitions implementing iso 14971 initiating risk management and design controls part 1: Web the purpose of this procedure is to describe the risk management.
Iso14971 Risk Management Template / Risk Management Procedure
Web the method for the evaluation of the overall residual risk and the criteria for its acceptability are required to be defined in the risk management plan. Web iso 14971 specifies a process through which the manufacturer of a medical device can identify hazards associated with a medical device, estimate and evaluate the risks. Web iso 14971:2019 medical devices —.
Iso14971 Risk Management Template Third edition of ISO 14971
Web the risk management process described in the new iso 14971 consists of several steps: For the particular medical device being considered, the. A new requirement to establish a method to evaluate the overall residual risk and criteria for. This document could be used as guidance in developing and maintaining a risk management. Risk management activities shall be planned.
What is new in ISO 149712019 Medical Device HQ
Web risk management plan template , which can be used as starting point for the practical implementation of the risk management process; Web iso 14971 specifies a process through which the manufacturer of a medical device can identify hazards associated with a medical device, estimate and evaluate the risks. Web iso 14971:2019 medical devices — application of risk management to.
ISO 149712019 Update for Risk Management Process
Web iso 14971 specifies a process through which the manufacturer of a medical device can identify hazards associated with a medical device, estimate and evaluate the risks. Web an iso 14971 checklist is a form based on the guidelines of iso 14971, a voluntary international standard that details how to apply risk management practices for. For the particular medical device.
ISO 149712019 Changes in the Current Version of ISO 14971 Oriel
For the particular medical device being considered, the. Web recently, the third version of iso 14971:2019 series has been notified and several aspects of this regulation include the best objectives to be achieved by the. Web iso 14971:2019 medical devices — application of risk management to medical devices abstract preview this document specifies terminology, principles and a process for risk..
ISO 149712019 Basics of Medical Device Risk Management
This document could be used as guidance in developing and maintaining a risk management. Scope of responsibilities and the individual phases. Risk management activities shall be planned. Click here to get this previously confidential risk management plan template in accordance with the requirements of iso 14971:2019 or. Web established principles of risk management that have evolved over many years.
Medical Device Risk Management Updates What is New in ISO 149712019?
Oliver eidel template download this is a free template,. Scope of responsibilities and the individual phases. Web risk management plan template , which can be used as starting point for the practical implementation of the risk management process; Web the method for the evaluation of the overall residual risk and the criteria for its acceptability are required to be defined.
Iso 14971 Risk Management Plan Example
Web iso 14971:2019 medical devices — application of risk management to medical devices abstract preview this document specifies terminology, principles and a process for risk. The focus of this blog post is the first of these six steps: Web the method for the evaluation of the overall residual risk and the criteria for its acceptability are required to be defined.
ISO 14971 Risk Management for Medical Devices The Definitive Guide
Web what's new in iso 14971:2019 key definitions implementing iso 14971 initiating risk management and design controls part 1: This document could be used as guidance in developing and maintaining a risk management. Web iso 14971:2019 medical devices — application of risk management to medical devices abstract preview this document specifies terminology, principles and a process for risk. Web templates.
Web The Risk Management Process Described In The New Iso 14971 Consists Of Several Steps:
Web templates iso 14971 templates updated june 20, 2023 template: Web an iso 14971 checklist is a form based on the guidelines of iso 14971, a voluntary international standard that details how to apply risk management practices for. Web iso 14971:2019 is the international standard for risk management in medical devices. Scope of responsibilities and the individual phases.
Risk Management Activities Shall Be Planned.
This document could be used as guidance in developing and maintaining a risk management. Web the risk management plan. For the particular medical device being considered, the. Web the purpose of this procedure is to describe the risk management process in accordance with iso 14971.
A New Requirement To Establish A Method To Evaluate The Overall Residual Risk And Criteria For.
Web recently, the third version of iso 14971:2019 series has been notified and several aspects of this regulation include the best objectives to be achieved by the. Web iso 14971:2019 medical devices — application of risk management to medical devices abstract preview this document specifies terminology, principles and a process for risk. The focus of this blog post is the first of these six steps: Web iso 14971 specifies a process through which the manufacturer of a medical device can identify hazards associated with a medical device, estimate and evaluate the risks.
Web Risk Management Plan Template , Which Can Be Used As Starting Point For The Practical Implementation Of The Risk Management Process;
Oliver eidel template download this is a free template,. Web in iso 14971:2019, section 4.4, the standard states that: Web established principles of risk management that have evolved over many years. Iso 14971:2019 has been recognized as the consensus standard by.