Acids React With Bases To Form Salt And Water
Acids React With Bases To Form Salt And Water - Web question a base reacts with an acid to form salt and water. Salts are odourless and have a salty taste, and many are soluble in water. Web solution verified by toppr correct option is a) when acids react with base, they neutralize each other's effect and thus it is called as neutralization reaction, energy is released. H 2 so 4 (aq) + cuo(s) →. Bases that are soluble in. This means that metal oxides and metal hydroxides are bases. Acid + base → salt + water. Web a salt and water are produced when acids react with metal oxides. Web a base is any substance that reacts with an acid to form a salt and water only. Web an acidic solution and a basic solution react together in a neutralization reaction that also forms a salt.
Web when an acid and a base are combined, water and a salt are the products. When acids react with a base, a salt and water are made. Web a neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. Web a base is any substance that reacts with an acid to form a salt and water only. Web solution verified by toppr correct option is a) when acids react with base, they neutralize each other's effect and thus it is called as neutralization reaction, energy is released. Web answer (1 of 4): Web acids react with bases to form a salt and water. Salts are ionic compounds containing a positive ion other than h + and a negative ion. Nitric acid + magnesium oxide → magnesium nitrate + water. Acid + base → salt + water.
A oxidation reaction b neutralisation reaction c reduction reaction d ionisation reaction. Web solution verified by toppr correct option is a) when acids react with base, they neutralize each other's effect and thus it is called as neutralization reaction, energy is released. Nitric acid + magnesium oxide → magnesium nitrate + water. Web acids react with bases to form a salt and water. Web acids and bases react with metals. Bases that are soluble in. Acid + base → salt + water. Web a neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. As discussed previously, metals that are more active than. Web a base is any substance that reacts with an acid to form a salt and water only.
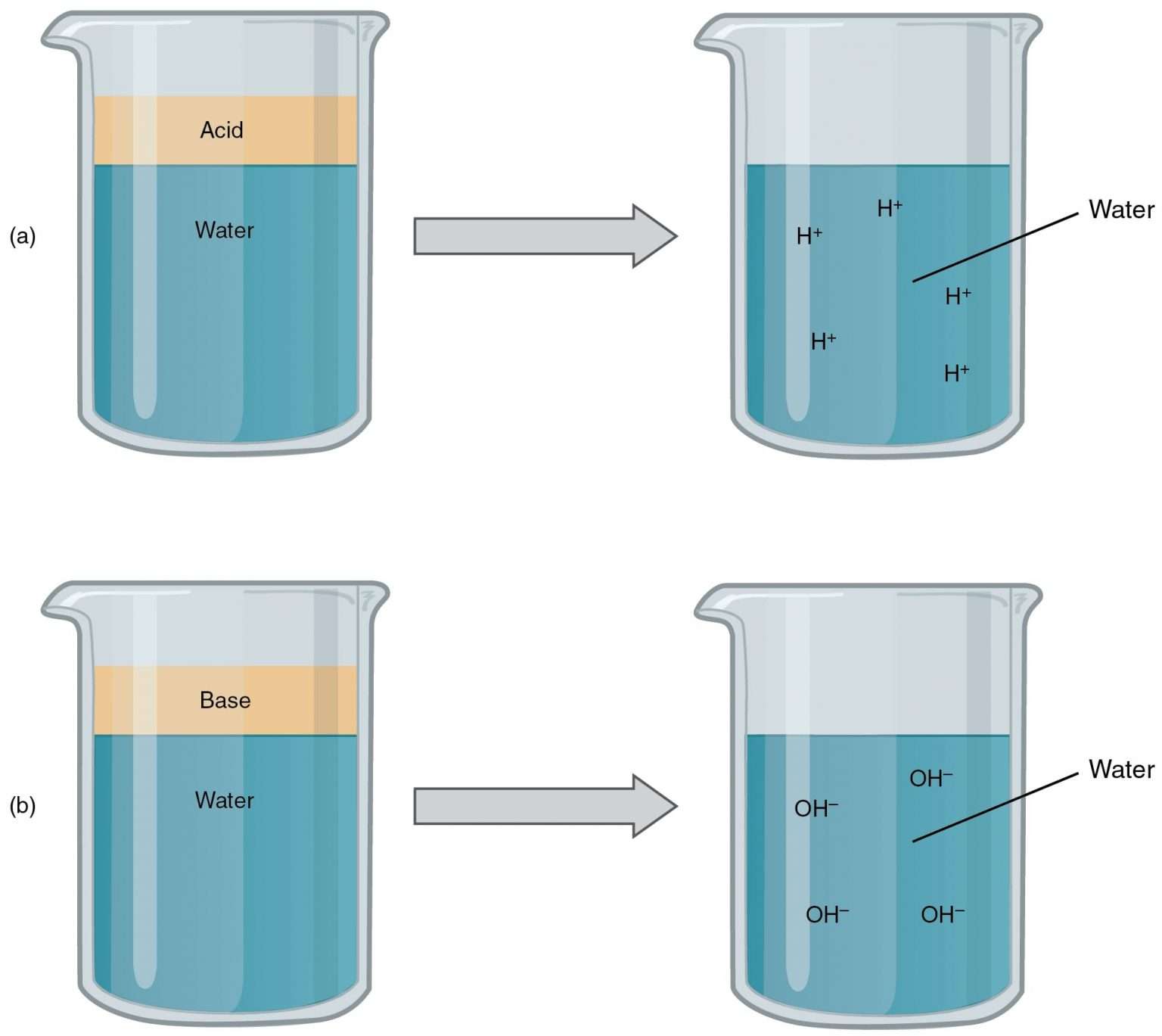
Acids Bases and Salts What happens when water is added to acid or
Sulfuric acid + copper(ii) oxide → copper(ii) sulfate + water. Salts are odourless and have a salty taste, and many are soluble in water. Metal oxides are bases, because they neutralise acids. Web answer (1 of 4): Bases that are soluble in.
Acids react with bases to form salt and water. This reaction is known as
Web an acidic solution and a basic solution react together in a neutralization reaction that also forms a salt. Bases that are soluble in. A oxidation reaction b neutralisation reaction c reduction reaction d ionisation reaction. For example, sodium hydroxide and hydrochloric acid react to form sodium. Web acids and bases react with metals.
CBSE Chemistry 7th Acids bases and Salts [Solved] CBSE ADDA
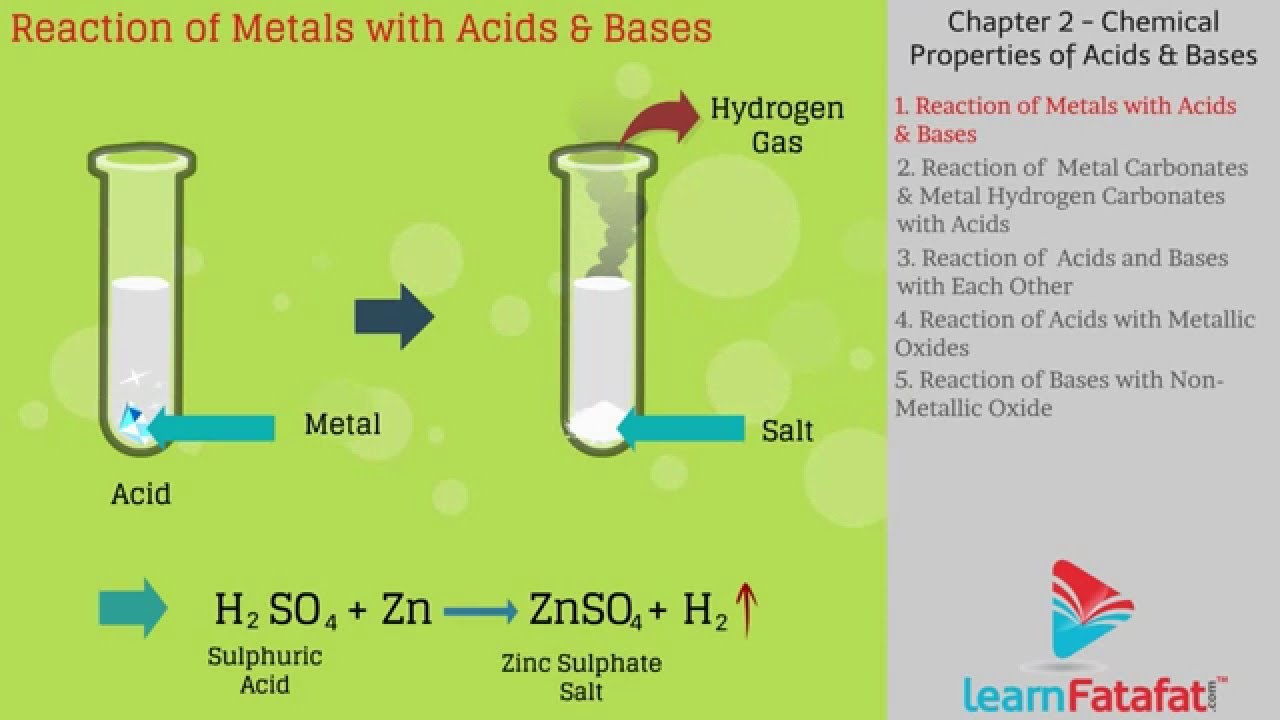
When acids react with a base, a salt and water are made. Web an acidic solution and a basic solution react together in a neutralization reaction that also forms a salt. Acids react with most metals to form a salt and hydrogen gas. Web a neutralization reaction can be defined as a chemical reaction in which an acid and base.
Acids, bases and salts CPD RSC Education
Web answer (1 of 4): As discussed previously, metals that are more active than. When acids react with a base, a salt and water are made. Web acids and bases react with metals. Acid + base → salt + water this is perhaps one of the most famous word equations [1] that can help remember chemical.
Lecture 19.1a Acid/Base Properties
Acid + base → salt + water this is perhaps one of the most famous word equations [1] that can help remember chemical. Web acids react with bases to form a salt and water. Nitric acid + magnesium oxide → magnesium nitrate + water. Web solution verified by toppr correct option is a) when acids react with base, they neutralize.
Chapter 8 Reactions in Aqueous Solution
Web when an acid and a base are placed together, they react to neutralize the acid and base properties, producing a salt. Web acids and bases react with metals. Acid + base → salt + water this is perhaps one of the most famous word equations [1] that can help remember chemical. Web question a base reacts with an acid.
Acid Bases and Salts Class 10 Science CBSE Chemical Properties of
For example, sodium hydroxide and hydrochloric acid react to form sodium. Web question a base reacts with an acid to form salt and water. Acid + base → salt + water this is perhaps one of the most famous word equations [1] that can help remember chemical. You are missing a couple of symbols: Acid + metal oxide → salt.
Acids and Bases
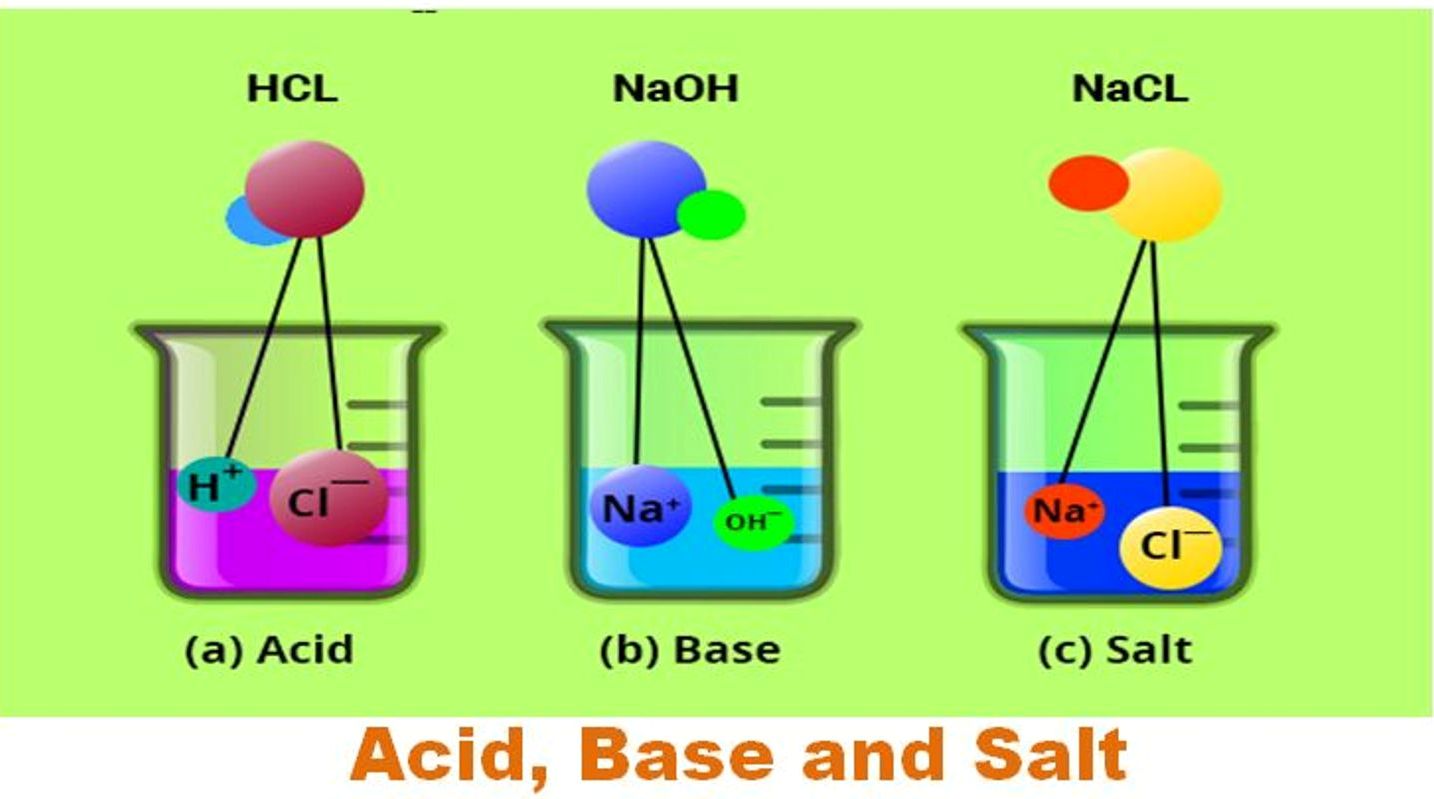
Salts are ionic compounds containing a positive ion other than h + and a negative ion. Web acid + base = salt + water when acids react with bases, they produce a salt and water. Web a base is any substance that reacts with an acid to form a salt and water only. Web acids and bases react with metals..
May 2020 Scienceandsf A Blog Published by Robert A. Lawler
Web acid + base = salt + water when acids react with bases, they produce a salt and water. Sulfuric acid + copper(ii) oxide → copper(ii) sulfate + water. H 2 so 4 (aq) + cuo(s) →. As discussed previously, metals that are more active than. When a seed (acid) played her bass (base) drum she became very upset and.
Acids and Bases 9 Properties, Useful Reaction & Examples
Web neutralisation is a reaction between an acid and an alkali that forms a salt and water. Salts are ionic compounds containing a positive ion other than h + and a negative ion. Nitric acid + magnesium oxide → magnesium nitrate + water. Web when an acid and a base are placed together, they react to neutralize the acid and.
You Are Missing A Couple Of Symbols:
Nitric acid + magnesium oxide → magnesium nitrate + water. Web question a base reacts with an acid to form salt and water. Web when an acid and a base are placed together, they react to neutralize the acid and base properties, producing a salt. Web solution verified by toppr correct option is a) when acids react with base, they neutralize each other's effect and thus it is called as neutralization reaction, energy is released.
Web Acids React With Bases To Form A Salt And Water.
When a seed (acid) played her bass (base) drum she became very upset and began to. Web acid + base = salt + water when acids react with bases, they produce a salt and water. When acids react with a base, a salt and water are made. A oxidation reaction b neutralisation reaction c reduction reaction d ionisation reaction.
Sulfuric Acid + Copper(Ii) Oxide → Copper(Ii) Sulfate + Water.
Acid + base → salt + water. As discussed previously, metals that are more active than. Metal oxides are bases, because they neutralise acids. Web a base is any substance that reacts with an acid to form a salt and water only.
Salts Are Odourless And Have A Salty Taste, And Many Are Soluble In Water.
Web an acidic solution and a basic solution react together in a neutralization reaction that also forms a salt. Web acids and bases react with metals. Web a neutralization reaction can be defined as a chemical reaction in which an acid and base quantitatively react together to form a salt and water as products. H 2 so 4 (aq) + cuo(s) →.


![CBSE Chemistry 7th Acids bases and Salts [Solved] CBSE ADDA](https://1.bp.blogspot.com/-F3SKPLqiR9g/UZ5HGgkzuiI/AAAAAAAABZU/tCoRRXbdkgs/s1600/aCIDS+bASE+AND+sALTS.png)