Alpha Helix And Beta Pleated Sheet
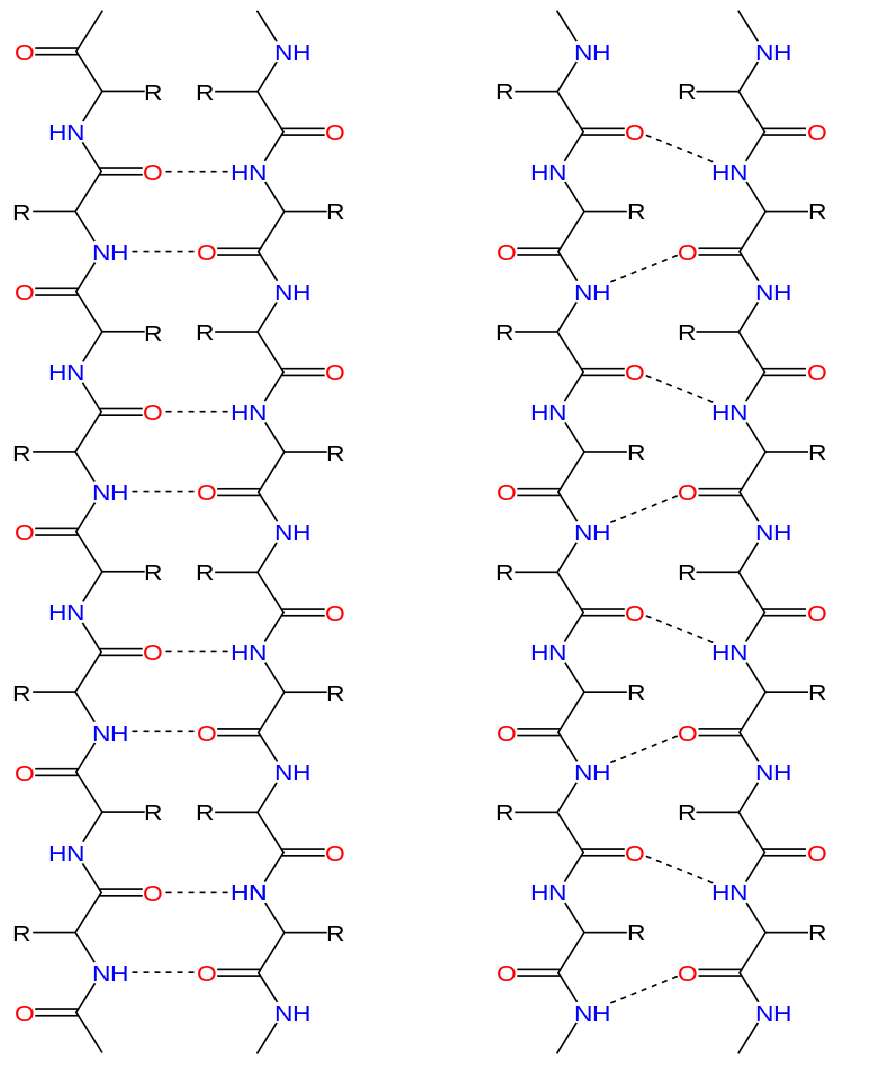
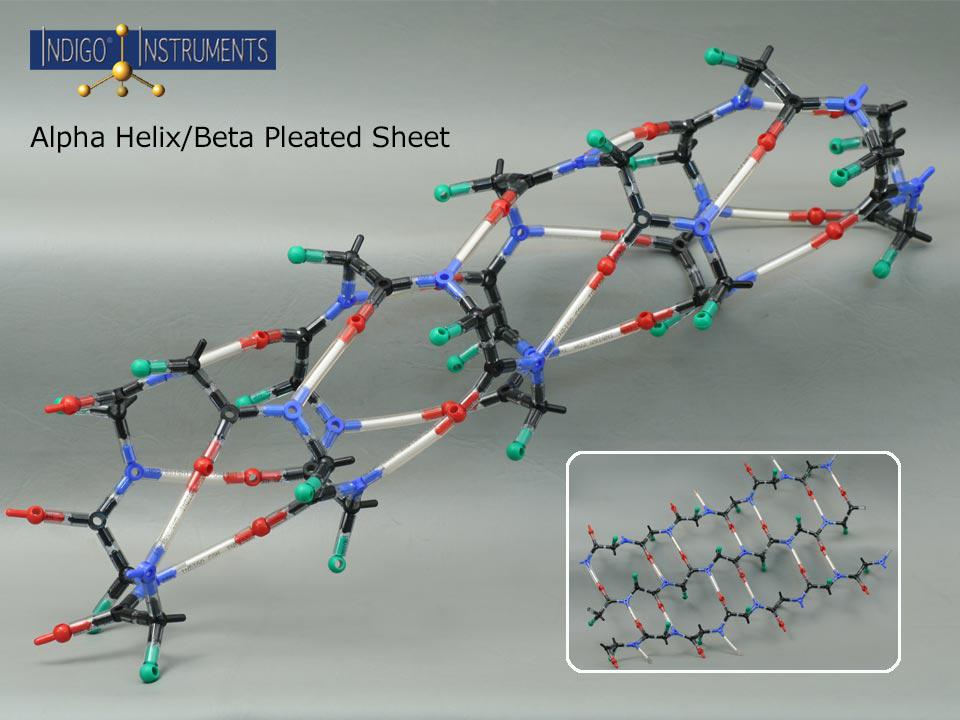
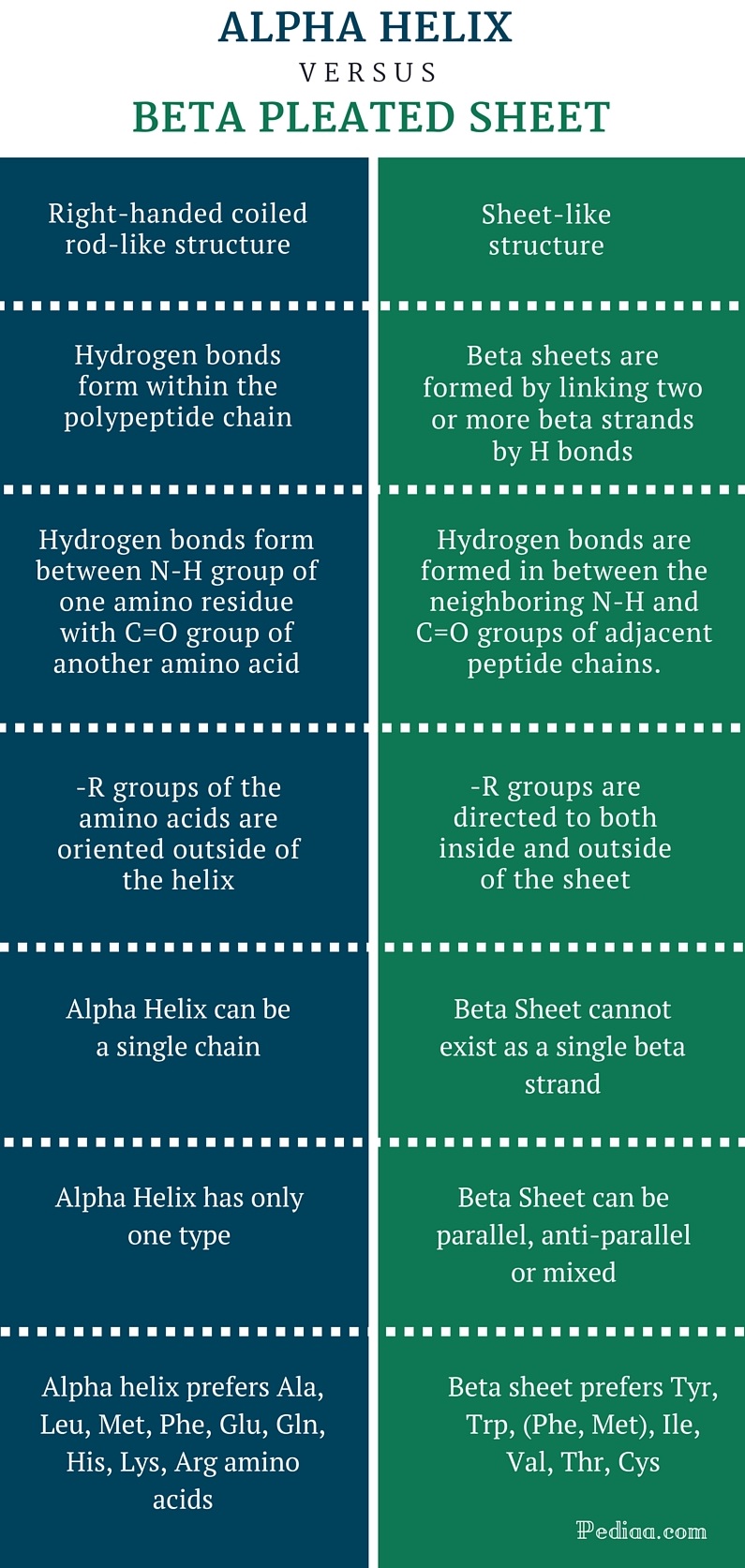
Alpha Helix And Beta Pleated Sheet - They both are shaped by hydrogen bonding between the carbonyl o of one amino acid and the amino h of another. This page explains how amino acids combine to make proteins and what is meant by the primary, secondary and tertiary structures. The tertiary structure of proteins; Web the most common types of secondary structures are the α helix and the β pleated sheet. The other portions of the polymer backbone that are regular but not repetitive are. Both structures are held in shape by hydrogen bonds, which form between the carbonyl o of one amino acid and the.
They both are shaped by hydrogen bonding between the carbonyl o of one amino acid and the amino h of another. Both structures are held in shape by hydrogen bonds, which form between the carbonyl o of one amino acid and the. Web the most common types of secondary structures are the α helix and the β pleated sheet. This page explains how amino acids combine to make proteins and what is meant by the primary, secondary and tertiary structures. The tertiary structure of proteins; The other portions of the polymer backbone that are regular but not repetitive are.
They both are shaped by hydrogen bonding between the carbonyl o of one amino acid and the amino h of another. The tertiary structure of proteins; The other portions of the polymer backbone that are regular but not repetitive are. Web the most common types of secondary structures are the α helix and the β pleated sheet. Both structures are held in shape by hydrogen bonds, which form between the carbonyl o of one amino acid and the. This page explains how amino acids combine to make proteins and what is meant by the primary, secondary and tertiary structures.
Proteins Microbiology
They both are shaped by hydrogen bonding between the carbonyl o of one amino acid and the amino h of another. The other portions of the polymer backbone that are regular but not repetitive are. Both structures are held in shape by hydrogen bonds, which form between the carbonyl o of one amino acid and the. This page explains how.
Difference Between Alpha Helix and Beta Pleated Sheet
The tertiary structure of proteins; Both structures are held in shape by hydrogen bonds, which form between the carbonyl o of one amino acid and the. They both are shaped by hydrogen bonding between the carbonyl o of one amino acid and the amino h of another. Web the most common types of secondary structures are the α helix and.
Difference Between Alpha Helix and Beta Pleated Sheet
Web the most common types of secondary structures are the α helix and the β pleated sheet. They both are shaped by hydrogen bonding between the carbonyl o of one amino acid and the amino h of another. The other portions of the polymer backbone that are regular but not repetitive are. Both structures are held in shape by hydrogen.
Alpha Helix Structure Molecular Model Kit Complements 12 Base Pair DNA
Both structures are held in shape by hydrogen bonds, which form between the carbonyl o of one amino acid and the. They both are shaped by hydrogen bonding between the carbonyl o of one amino acid and the amino h of another. This page explains how amino acids combine to make proteins and what is meant by the primary, secondary.
Difference Between Alpha Helix and Beta Pleated Sheet Compare the
Web the most common types of secondary structures are the α helix and the β pleated sheet. The tertiary structure of proteins; They both are shaped by hydrogen bonding between the carbonyl o of one amino acid and the amino h of another. The other portions of the polymer backbone that are regular but not repetitive are. Both structures are.
Difference Between Alpha Helix and Beta Pleated Sheet
Web the most common types of secondary structures are the α helix and the β pleated sheet. They both are shaped by hydrogen bonding between the carbonyl o of one amino acid and the amino h of another. The tertiary structure of proteins; This page explains how amino acids combine to make proteins and what is meant by the primary,.
Difference Between Alpha Helix and Beta Pleated Sheet Compare the
Both structures are held in shape by hydrogen bonds, which form between the carbonyl o of one amino acid and the. This page explains how amino acids combine to make proteins and what is meant by the primary, secondary and tertiary structures. Web the most common types of secondary structures are the α helix and the β pleated sheet. The.
Secondary structures of keratin protein (beta pleated sheets and alpha
This page explains how amino acids combine to make proteins and what is meant by the primary, secondary and tertiary structures. Web the most common types of secondary structures are the α helix and the β pleated sheet. The tertiary structure of proteins; The other portions of the polymer backbone that are regular but not repetitive are. Both structures are.
College. Science. Life Essential Cell Biology 3rd Ch 4 Protein
The other portions of the polymer backbone that are regular but not repetitive are. Web the most common types of secondary structures are the α helix and the β pleated sheet. This page explains how amino acids combine to make proteins and what is meant by the primary, secondary and tertiary structures. They both are shaped by hydrogen bonding between.
[Solved] How many hydrogen bonds involving the backbone CO and NH can
They both are shaped by hydrogen bonding between the carbonyl o of one amino acid and the amino h of another. The other portions of the polymer backbone that are regular but not repetitive are. The tertiary structure of proteins; Both structures are held in shape by hydrogen bonds, which form between the carbonyl o of one amino acid and.
Both Structures Are Held In Shape By Hydrogen Bonds, Which Form Between The Carbonyl O Of One Amino Acid And The.
Web the most common types of secondary structures are the α helix and the β pleated sheet. They both are shaped by hydrogen bonding between the carbonyl o of one amino acid and the amino h of another. The other portions of the polymer backbone that are regular but not repetitive are. The tertiary structure of proteins;






![[Solved] How many hydrogen bonds involving the backbone CO and NH can](https://cdn.testbook.com/images/production/quesImages/qImage646f23abed4806260c7a04d2.png)