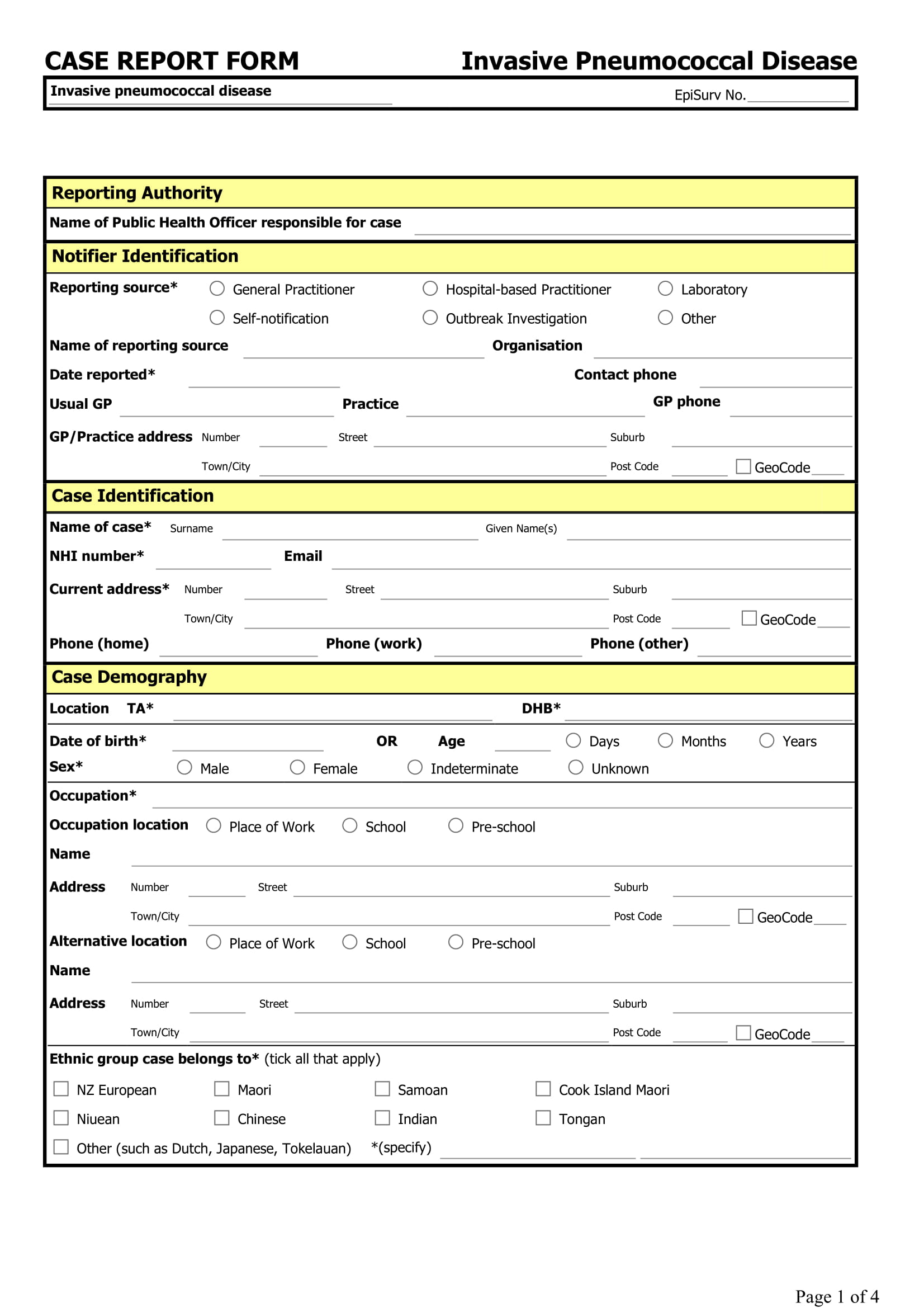
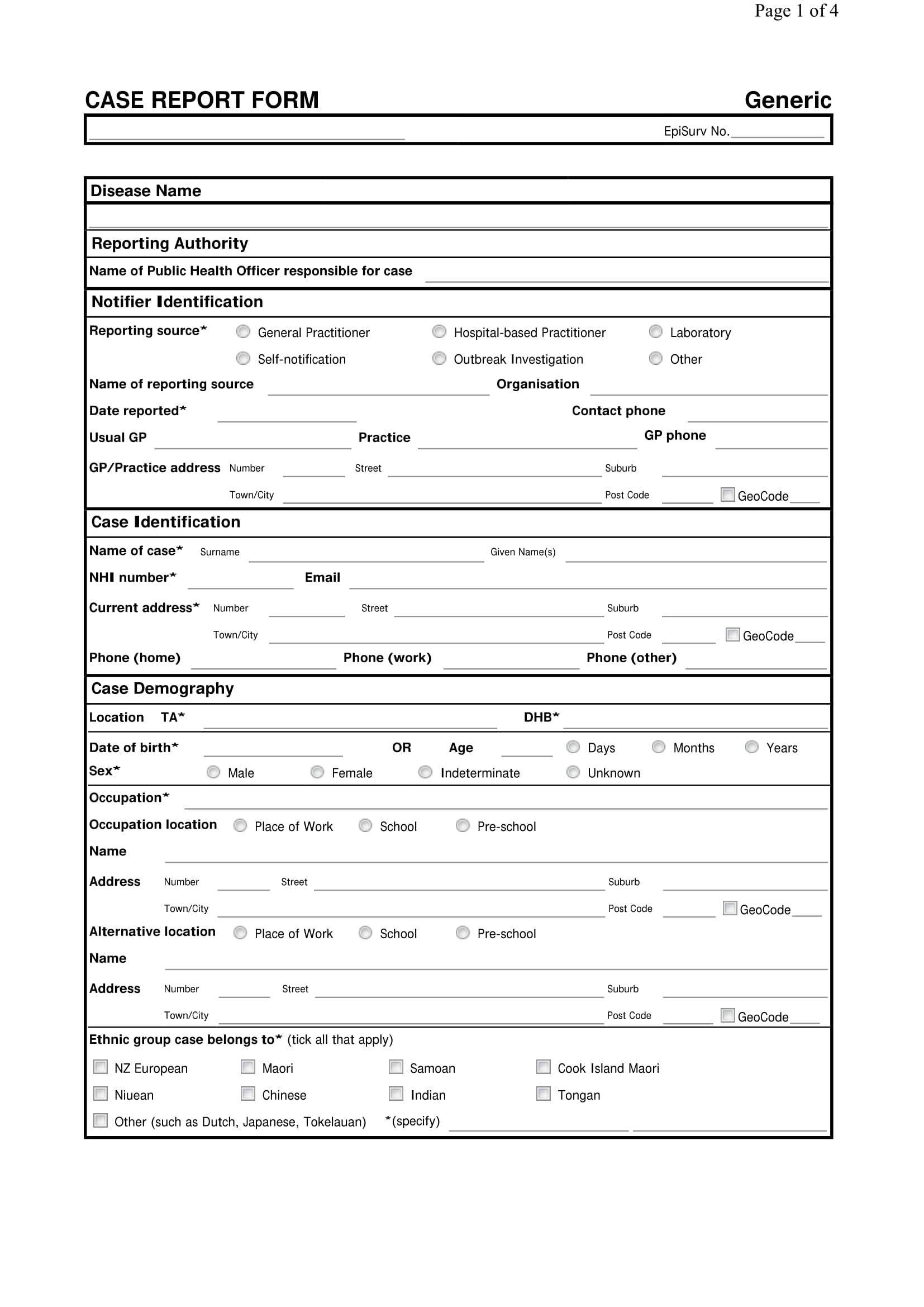
Case Report Form
Case Report Form - Web what is a case report form? Case reports usually describe an unusual or novel occurrence and as such, remain one of the cornerstones of medical progress and provide many new ideas in medicine. Though paper crfs are still used largely, use of electronic crfs (ecrfs) are gaining popularity due to the advantages they offer such as improved data. Each clinical trial participant has a. [ 1] site personnel capture the subject's data on the crf, which is collected during their participation in a clinical trial. But they are a key component for recording the data in a clinical trial. Web a case report form (crf) is designed to collect the patient data in a clinical trial; Web a case report form (or crf) is a paper or electronic questionnaire specifically used in clinical trial research. Epix notification of travelers meets clinical criteria and epidemiologic evidence with no confirmatory lab testing* Most of the time, participants in clinical research are not even aware of crfs.
The crf facilitates complete and standardized data collection that promotes efficient processing, analysis, and reporting of information, as well as exchange of data across. Case report forms (crfs) are arguably the most important documentation in a clinical trial since they are the last point of data entry, which ultimately influences the outcome of a study. Web what is a case report form? Web a case report form (or crf) is a paper or electronic questionnaire specifically used in clinical trial research. The cdash standards identify those elements that should be captured on a case report form (crf). All data on each patient participating in a clinical trial are held and/or documented in the crf. Case report form (crf) is a specialized document in clinical research. Each clinical trial participant has a. Case reports usually describe an unusual or novel occurrence and as such, remain one of the cornerstones of medical progress and provide many new ideas in medicine. Web a case report form (crf) is designed to collect the patient data in a clinical trial;
Its development represents a significant part of the clinical trial and can affect study success. [ 1] site personnel capture the subject's data on the crf, which is collected during their participation in a clinical trial. Web a case report form (or crf) is a paper or electronic questionnaire specifically used in clinical trial research. Case reports usually describe an unusual or novel occurrence and as such, remain one of the cornerstones of medical progress and provide many new ideas in medicine. A case report form is an essential tool for clinical research, although it has been extended for use in other areas of medicine. All data on each patient participating in a clinical trial are held and/or documented in the crf. Case report form (crf) is a specialized document in clinical research. The crf facilitates complete and standardized data collection that promotes efficient processing, analysis, and reporting of information, as well as exchange of data across. These templates are consistent with the fda cdash (clinical data acquisition standards harmonization) standards. Web a case report form (crf) is designed to collect the patient data in a clinical trial;
10+ Case Report Templates PDF, Word, Pages Free & Premium Templates
Web what is a case report form? Research data is ultimately submitted to the sponsor and/or analyzed by the emory investigator by either paper case report forms (crfs) or. Case report form (crf) is a specialized document in clinical research. [1] the case report form is the tool used by the sponsor of the clinical trial to collect data from.
FREE 15+ Case Report Forms in PDF MS Word
Though paper crfs are still used largely, use of electronic crfs (ecrfs) are gaining popularity due to the advantages they offer such as improved data. Its development represents a significant part of the clinical trial and can affect study success. [1] the case report form is the tool used by the sponsor of the clinical trial to collect data from.
Case Report Form Case Report Format
Case report form (crf) is a specialized document in clinical research. Web case classification and identification what is the current status of this person? Web a case report form (crf) is designed to collect the patient data in a clinical trial; Each clinical trial participant has a. These templates are consistent with the fda cdash (clinical data acquisition standards harmonization).
FREE 15+ Case Report Forms in PDF MS Word
Web case report forms (or crfs, for short) are an integral component of clinical trials. The cdash standards identify those elements that should be captured on a case report form (crf). It should be study protocol driven, robust in content and have material to collect the study specific data. Epix notification of travelers meets clinical criteria and epidemiologic evidence with.
FREE 15+ Case Report Forms in PDF MS Word
Web a case report form (or crf) is a paper or electronic questionnaire specifically used in clinical trial research. [1] the case report form is the tool used by the sponsor of the clinical trial to collect data from each participating patient. Case reports usually describe an unusual or novel occurrence and as such, remain one of the cornerstones of.
Free 15+ Case Report Forms In Pdf Ms Word Inside Case Report Form
Web a case report form (or crf) is a paper or electronic questionnaire specifically used in clinical trial research. But they are a key component for recording the data in a clinical trial. Web case report forms (or crfs, for short) are an integral component of clinical trials. Web a case report form (crf) is designed to collect the patient.
FREE 15+ Case Report Forms in PDF MS Word
Epix notification of travelers meets clinical criteria and epidemiologic evidence with no confirmatory lab testing* Case report form (crf) is a specialized document in clinical research. Case report forms (crfs) are arguably the most important documentation in a clinical trial since they are the last point of data entry, which ultimately influences the outcome of a study. Web a case.
Blank Case Report Form RIAT Support Center
Case report forms (crfs) are arguably the most important documentation in a clinical trial since they are the last point of data entry, which ultimately influences the outcome of a study. [1] the case report form is the tool used by the sponsor of the clinical trial to collect data from each participating patient. The crf facilitates complete and standardized.
What Is a Case Report Form? [ Importance, Tips, Samples ]
Web case classification and identification what is the current status of this person? Web what is a case report form? These templates are consistent with the fda cdash (clinical data acquisition standards harmonization) standards. All data on each patient participating in a clinical trial are held and/or documented in the crf. Each clinical trial participant has a.
Blank Case Report Form RIAT Support Center
Web a case report form (or crf) is a paper or electronic questionnaire specifically used in clinical trial research. The cdash standards identify those elements that should be captured on a case report form (crf). Case reports usually describe an unusual or novel occurrence and as such, remain one of the cornerstones of medical progress and provide many new ideas.
Research Data Is Ultimately Submitted To The Sponsor And/Or Analyzed By The Emory Investigator By Either Paper Case Report Forms (Crfs) Or.
The crf facilitates complete and standardized data collection that promotes efficient processing, analysis, and reporting of information, as well as exchange of data across. Web case classification and identification what is the current status of this person? [1] the case report form is the tool used by the sponsor of the clinical trial to collect data from each participating patient. Web what is a case report form?
Case Report Form (Crf) Is A Specialized Document In Clinical Research.
Epix notification of travelers meets clinical criteria and epidemiologic evidence with no confirmatory lab testing* Case reports usually describe an unusual or novel occurrence and as such, remain one of the cornerstones of medical progress and provide many new ideas in medicine. Web case report forms (or crfs, for short) are an integral component of clinical trials. The cdash standards identify those elements that should be captured on a case report form (crf).
Its Development Represents A Significant Part Of The Clinical Trial And Can Affect Study Success.
But they are a key component for recording the data in a clinical trial. A case report form is an essential tool for clinical research, although it has been extended for use in other areas of medicine. Though paper crfs are still used largely, use of electronic crfs (ecrfs) are gaining popularity due to the advantages they offer such as improved data. Web a case report form (crf) is designed to collect the patient data in a clinical trial;
Case Report Forms (Crfs) Are Arguably The Most Important Documentation In A Clinical Trial Since They Are The Last Point Of Data Entry, Which Ultimately Influences The Outcome Of A Study.
These templates are consistent with the fda cdash (clinical data acquisition standards harmonization) standards. [ 1] site personnel capture the subject's data on the crf, which is collected during their participation in a clinical trial. Most of the time, participants in clinical research are not even aware of crfs. Each clinical trial participant has a.




![What Is a Case Report Form? [ Importance, Tips, Samples ]](https://images.sampleforms.com/wp-content/uploads/2018/02/Confidential-Case-Report-Form-in-PDF-1.jpg)
