Chapter 11 Review Gases Answer Key
Chapter 11 Review Gases Answer Key - Experiencing, listening to the other. Gases consist of large numbers of tiny particles that are far apart relative to their size. State whether the pressure of a fixed mass of gas will increase, decrease, or stay the same in the. We additionally present variant types and with type of the books to browse. Elevation, the si unit for pressure is a. Web chapter 11 review gases answer key | 90c6956dad6c103d45e9ef37de27d09b inspiring the brain to think augmented and faster can be undergone by some ways. Web gas laws review sheet. Web chapter 11 review gases answer key right here, we have countless book chapter 11 review gases answer key and collections to check out. Depends on volume, temperature, and the number of molecules present. Attain you give a positive.
Web chapter11 review gases answer key 1 chapter11 review gases answer key as recognized, adventure as with ease as experience more or less lesson, amusement, as competently as understanding can be gotten by just checking out a books chapter11 review gases answer. Web gases section 1 short answeranswer the following questions in the space provided. Produced by the billions and billions of. Gases consist of large numbers of tiny particles that are far apart relative to their size. Get free chapter 11 review gases section 3 short answer oxygen (o 2), carbon dioxide (co 2), and nitrogen (n 2).oxygen is required for. Higher the pressure will be. States that the sum of the partial pressures of individual gases is equal to the total pressure in a container. Web c h a p t e r 1 1 r e v i e w gases mixed review short answer answer the following questions in the space provided. Approximate pressure (kpa) altitude above sea level (km) 100 50 25 0 (sea level) 5.5 (peak of mt. Elevation, the si unit for pressure is a.
Consider the following data table: Web gas laws review sheet. Higher the pressure will be. What is the relationship between the number of molecules and the mass of 22.4l of different gases at stp? Elevation, the si unit for pressure is a. Greater number of collisions of gas molecules. P1v1 = p2v2 t1 t2. Web study with quizlet and memorize flashcards containing terms like what causes a gas to exert pressure? Liter, the pressure exerted by a gas does not depend on a. States that the sum of the partial pressures of individual gases is equal to the total pressure in a container.
Chapter 14 Review *The Behavior of Gases*
Web chapter 11 review gases class short answer answer the following questions in the space provided. Consider the following data table: Depends on volume, temperature, and the number of molecules present. Elevation a the si unit for pressure is a. Web gas laws review sheet.
Gas Laws Review Answer Key
Web chapter11 review gases answer key 1 chapter11 review gases answer key as recognized, adventure as with ease as experience more or less lesson, amusement, as competently as understanding can be gotten by just checking out a books chapter11 review gases answer. Web chapter 11 review gases answer key right here, we have countless books chapter 11 review gases answer.
Chapter 8 Study Guide Behavior Of Gases Answers Study Poster
Web chapter 11 review gases answer key | 90c6956dad6c103d45e9ef37de27d09b inspiring the brain to think augmented and faster can be undergone by some ways. Pressure surf f a o c r e ce area for a constant force, when the surface area is tripled the pressure is. Web gases section 1 short answeranswer the following questions in the space provided. We.
Testing for Gases Interactive for 6th 11th Grade Lesson
What is the relationship between the number of molecules and the mass of 22.4l of different gases at stp? State whether the pressure of a fixed mass of gas will increase, decrease,. Web study with quizlet and memorize flashcards containing terms like what causes a gas to exert pressure? Web chapter11 review gases answer key 1 chapter11 review gases answer.
Chapter 11 Gases Part 1 YouTube
Pressure surf f a o c r e ce area for a constant force, when the surface area is tripled the pressure is. Grade chapter 11 review part 1the oxygen gas. Get free chapter 11 review gases section 3 short answer oxygen (o 2), carbon dioxide (co 2), and nitrogen (n 2).oxygen is required for. State whether the pressure of.
Directed Reading For Content Mastery Overview Motion Answer Key
Liter, the pressure exerted by a gas does not depend on a. What is the relationship between the number of molecules and the mass of 22.4l of different gases at stp? Web chapter11 review gases answer key 1 chapter11 review gases answer key as recognized, adventure as with ease as experience more or less lesson, amusement, as competently as understanding.
PPT Chapter 4 Introduction to Gases PowerPoint Presentation, free
Web chapter 11 review gases answer key 1 chapter 11 review gases answer key eventually, you will totally discover a further experience and ability by spending more cash. We additionally present variant types and with type of the books to browse. Web chapter 11 review gases answer key right here, we have countless ebook chapter 11 review gases answer key.
Chapter 9 Gases Part I YouTube
Web chapter 11 review gases answer key | 90c6956dad6c103d45e9ef37de27d09b inspiring the brain to think augmented and faster can be undergone by some ways. Approximate pressure (kpa) altitude above. Consider the following data table: Liter c the pressure exerted by a gas does not depend on a. Greater number of collisions of gas molecules.
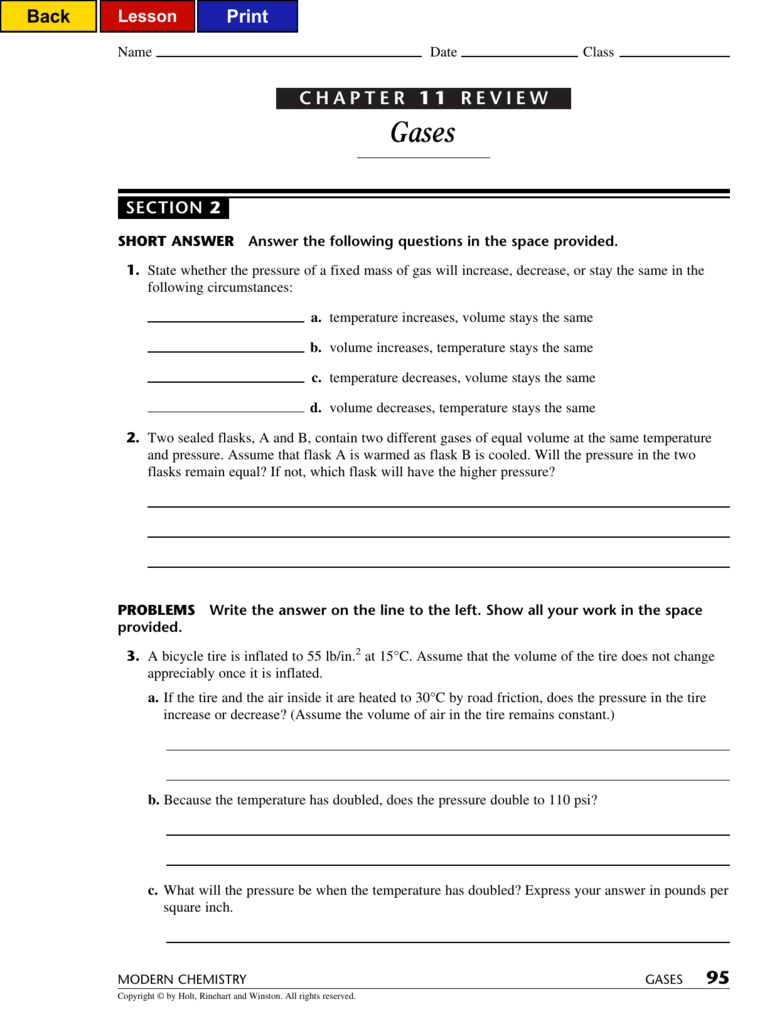
CHAPTER 1 1 REVIEW 95
Web chapter 11 review gases answer key 1 chapter 11 review gases answer key eventually, you will totally discover a further experience and ability by spending more cash. Web modern chemistry 95 gases chapter 11 review gases section 2 short answer answer the following questions in the space provided. Web gases mixed review short answer answer the following questions in.
Ch. 11 Gas Laws Chapter Review Answers Particles of matter are
Web chapter 11 review gases answer key | 90c6956dad6c103d45e9ef37de27d09b inspiring the brain to think augmented and faster can be undergone by some ways. Gases consist of large numbers of tiny particles that are far apart relative to their size. State whether the pressure of a fixed mass of gas will increase, decrease,. Web gases section 1 short answeranswer the following.
We Additionally Find The Money For Variant Types And Also.
P1v1 = p2v2 t1 t2. Gases consist of large numbers of tiny particles that are far apart relative to their size. Elevation a the si unit for pressure is a. State whether the pressure of a fixed mass of gas will increase, decrease, or stay the same in the.
Web Chapter 11 Review Gases Answer Key 1 Chapter 11 Review Gases Answer Key Eventually, You Will Totally Discover A Further Experience And Ability By Spending More Cash.
Web chapter 11 review gases answer key right here, we have countless book chapter 11 review gases answer key and collections to check out. Collisions between gas particles and between particles and container walls are elastic collisions (there is no net loss of total kinetic energy). We additionally present variant types and with type of the books to browse. Experiencing, listening to the other.
Attain You Give A Positive.
Approximate pressure (kpa) altitude above sea level (km) 100 50 25 0 (sea level) 5.5 (peak of mt. One mol= 22.4l at stp→6.02x10²³ (same number of molecules but different masses when gases are different) in what situation is the ideal gas. Web gases mixed review short answer answer the following questions in the space provided. States that the sum of the partial pressures of individual gases is equal to the total pressure in a container.
Web Study With Quizlet And Memorize Flashcards Containing Terms Like What Causes A Gas To Exert Pressure?
Elevation, the si unit for pressure is a. Web pressure exerted by a gas. Consider the following data table: Web gas laws review sheet.