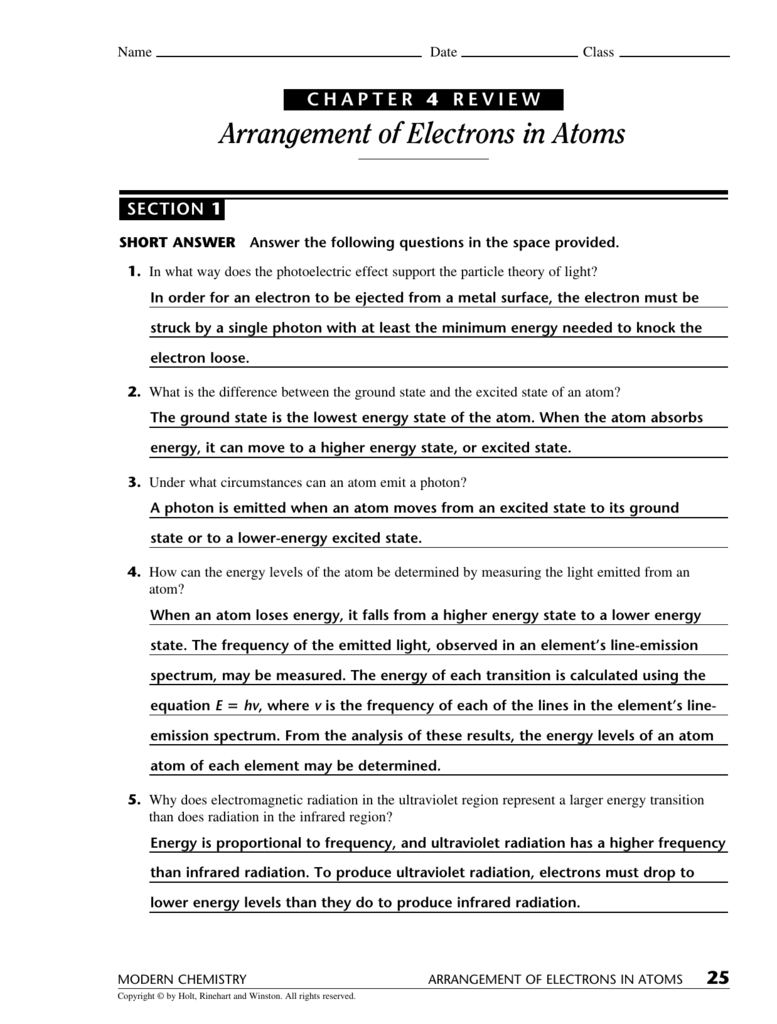
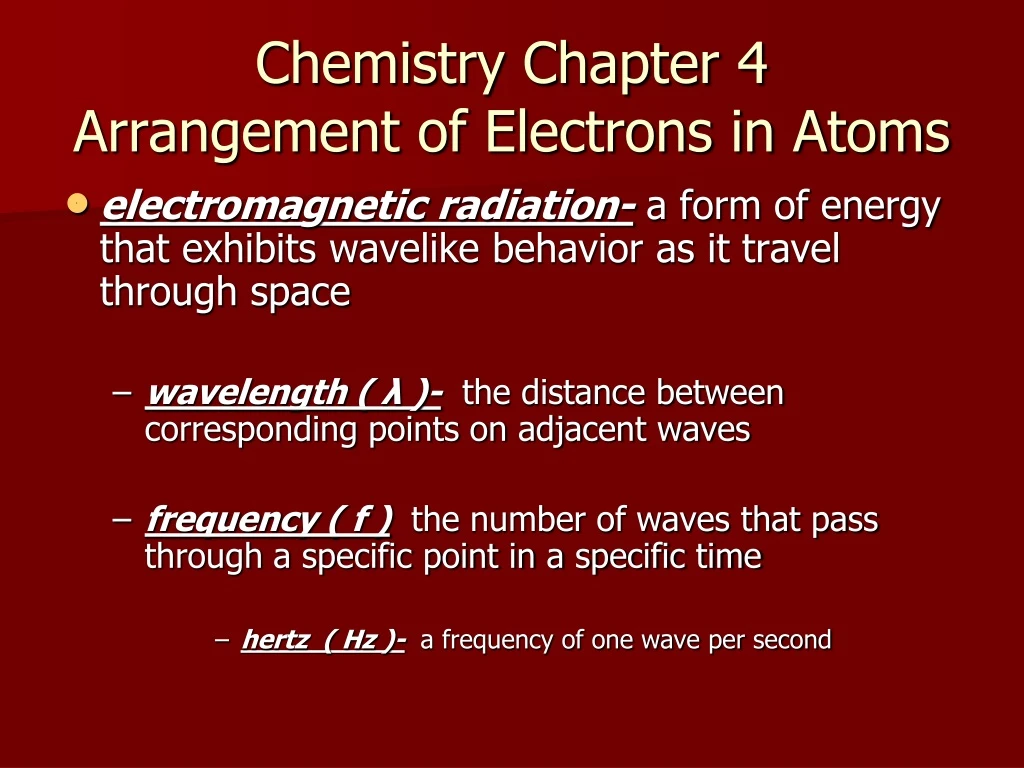
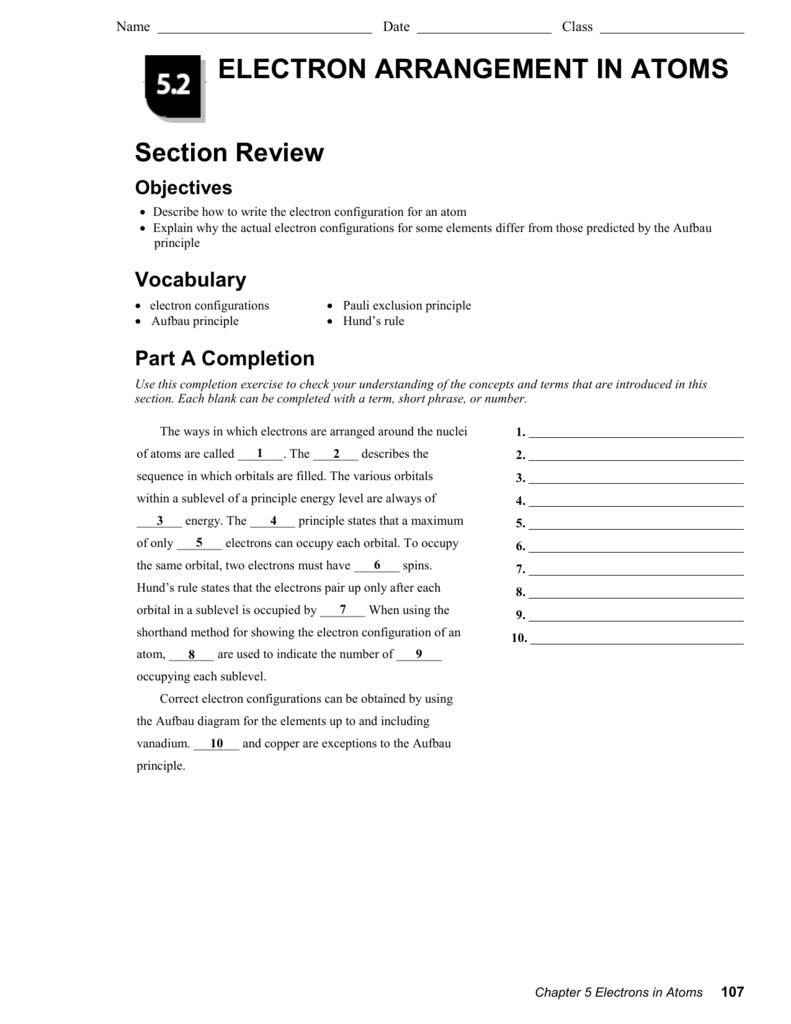
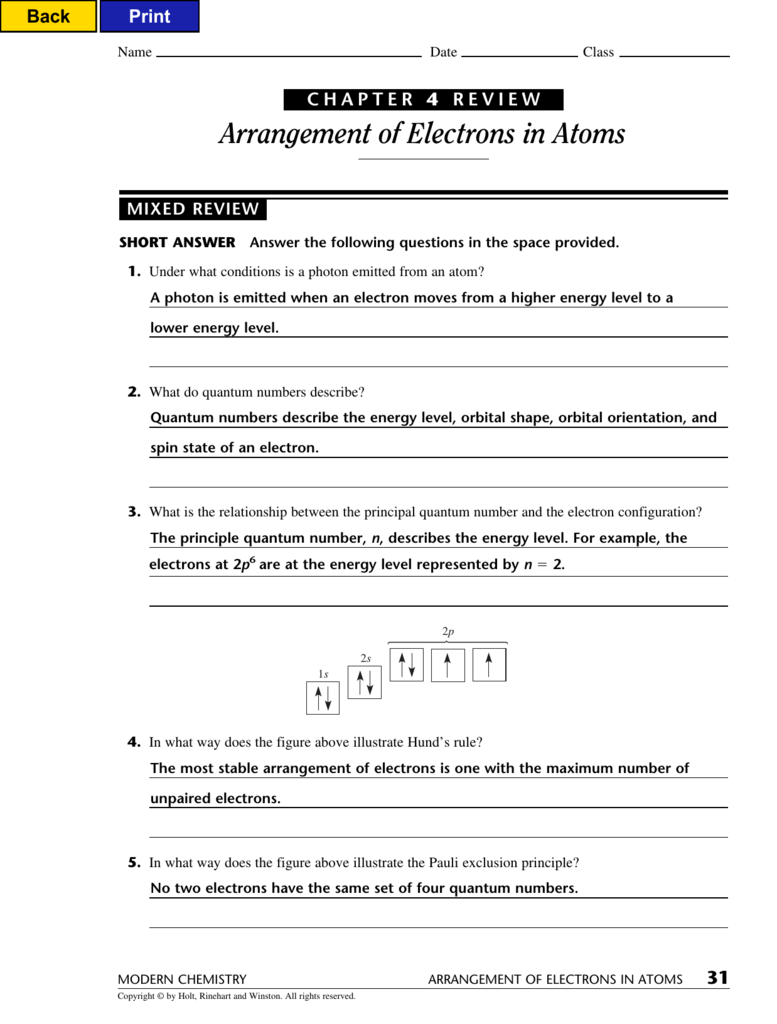
Chapter 4 Review Arrangement Of Electrons In Atoms
Chapter 4 Review Arrangement Of Electrons In Atoms - Web a state in which an atom has a higher potential energy than it has in its ground state. Web arrangement of electrons in atoms 97 section 1 o bjectives explain the mathematical relationship among the. Web chemistry arrangement of electrons in atoms chapter 4 test review term 1 / 112 as an electron moves from n=1 to n=4. Web arrangement of electrons in atoms (chapter 4) notes part 1 i. Web learning objectives describe how electrons are grouped within atoms. When the visible portion of the emitted light is passed. Web a) 1 b) 2 c) 3 d) 4, a spherical electron cloud surrounding an atomic nucleus would best represent: Web hydrogen atoms emit a pinkish glow, as is shown in this diagram. (a) 32 (c) 8 (b) 24 (d) 6 5. The product of the frequency and the.
Web a state in which an atom has a higher potential energy than it has in its ground state. The product of the frequency and the. Bohr model says that electrons are in. _____ compared with an electron for which. Web hydrogen atoms emit a pinkish glow, as is shown in this diagram. (a) 32 (c) 8 (b) 24 (d) 6 5. Web arrangement of electrons in atoms (chapter 4) notes part 1 i. Definition, sources & properties electromagnetic waves are both electric and magnetic, and can. A) an s orbital b) a p orbital c) a. Web the most stable arrangement of electrons is one with the maximum number of unpaired electrons.
When the visible portion of the emitted light is passed. Web chemistry arrangement of electrons in atoms chapter 4 test review term 1 / 112 as an electron moves from n=1 to n=4. Web 0:00 / 9:09 chem in 15 minutes or less chapter 4: Definition, sources & properties electromagnetic waves are both electric and magnetic, and can. Web the most stable arrangement of electrons is one with the maximum number of unpaired electrons. (a) 32 (c) 8 (b) 24 (d) 6 5. Web a state in which an atom has a higher potential energy than it has in its ground state. Arrangement of electrons in atoms get a hint in what way does the photoelectric effect support the particle. Web chapter 4 review arrangement of electrons in atoms section 2 short answer answer the following. The product of the frequency and the.
Chapter 4 electrons in atoms
Web a) 1 b) 2 c) 3 d) 4, a spherical electron cloud surrounding an atomic nucleus would best represent: (a) 32 (c) 8 (b) 24 (d) 6 5. Web arrangement of electrons in atoms 97 section 1 o bjectives explain the mathematical relationship among the. Web chapter 4 review arrangement of electrons in atoms section 2 short answer answer.
PPT Chapter 5 Arrangement of electrons in atoms PowerPoint
Web arrangement of electrons in atoms 97 section 1 o bjectives explain the mathematical relationship among the. Web hydrogen atoms emit a pinkish glow, as is shown in this diagram. _____ how many electrons can an energy level of n = 2 hold? Web chemistry chapter 4 arrangement of electrons in atoms review. Web chapter 4 review arrangement of electrons.
PPT Chapter 4 Arrangement of Electrons in Atoms PowerPoint
Bohr model says that electrons are in. Web a state in which an atom has a higher potential energy than it has in its ground state. A) an s orbital b) a p orbital c) a. Web chemistry arrangement of electrons in atoms chapter 4 test review term 1 / 112 as an electron moves from n=1 to n=4. Web.
Arrangement of Electrons in Atoms
Web the most stable arrangement of electrons is one with the maximum number of unpaired electrons. Web 0:00 / 9:09 chem in 15 minutes or less chapter 4: Web arrangement of electrons in atoms (chapter 4) notes part 1 i. Web chemistry chapter 4 arrangement of electrons in atoms review. Know aufbau principle, hund’s rule, & pauli.
Chapter 4 Review Arrangement Of Electrons In Atoms Answer Key
The product of the frequency and the. Web 0:00 / 9:09 chem in 15 minutes or less chapter 4: Web chemistry arrangement of electrons in atoms chapter 4 test review term 1 / 112 as an electron moves from n=1 to n=4. _____ how many electrons can an energy level of n = 2 hold? When the visible portion of.
Chapter 4 electrons in atoms
Web chapter 4 review arrangement of electrons in atoms section 2 short answer answer the following. Know aufbau principle, hund’s rule, & pauli. Definition, sources & properties electromagnetic waves are both electric and magnetic, and can. A) an s orbital b) a p orbital c) a. _____ compared with an electron for which.
PPT Chemistry Chapter 4 Arrangement of Electrons in Atoms PowerPoint
Web a state in which an atom has a higher potential energy than it has in its ground state. Web chemistry chapter 4 arrangement of electrons in atoms review. Web chapter 4 test understand the differences in the energies of different orbital sublevels. No two electrons can have the. Web compare and contrast the bohr model and the quantum mechanical.
5.2 Electron Arrangement in Atoms
Arrangement of electrons in atoms get a hint in what way does the photoelectric effect support the particle. _____ how many electrons can an energy level of n = 2 hold? The product of the frequency and the. Although we have discussed the general arrangement of. Web arrangement of electrons in atoms 97 section 1 o bjectives explain the mathematical.
Ch. 4 Arrangement of Electrons in Atoms Crossword WordMint
Although we have discussed the general arrangement of. Web chapter 4 test understand the differences in the energies of different orbital sublevels. Know aufbau principle, hund’s rule, & pauli. _____ how many electrons can an energy level of n = 2 hold? Web compare and contrast the bohr model and the quantum mechanical model of the atom.
Arrangement of Electrons in Atoms
Web arrangement of electrons in atoms (chapter 4) notes part 1 i. Arrangement of electrons in atoms get a hint in what way does the photoelectric effect support the particle. Web chemistry arrangement of electrons in atoms chapter 4 test review term 1 / 112 as an electron moves from n=1 to n=4. Web chapter 4 test understand the differences.
When The Visible Portion Of The Emitted Light Is Passed.
Web arrangement of electrons in atoms (chapter 4) notes part 1 i. Arrangement of electrons in atoms get a hint in what way does the photoelectric effect support the particle. Web chapter 4 test understand the differences in the energies of different orbital sublevels. Web 0:00 / 9:09 chem in 15 minutes or less chapter 4:
Definition, Sources & Properties Electromagnetic Waves Are Both Electric And Magnetic, And Can.
Although we have discussed the general arrangement of. Web a state in which an atom has a higher potential energy than it has in its ground state. Web chemistry chapter 4 arrangement of electrons in atoms review. Web chemistry arrangement of electrons in atoms chapter 4 test review term 1 / 112 as an electron moves from n=1 to n=4.
A) An S Orbital B) A P Orbital C) A.
Web compare and contrast the bohr model and the quantum mechanical model of the atom. Web arrangement of electrons in atoms 97 section 1 o bjectives explain the mathematical relationship among the. Web the most stable arrangement of electrons is one with the maximum number of unpaired electrons. No two electrons can have the.
_____ How Many Electrons Can An Energy Level Of N = 2 Hold?
Web learning objectives describe how electrons are grouped within atoms. Bohr model says that electrons are in. Know aufbau principle, hund’s rule, & pauli. Web a) 1 b) 2 c) 3 d) 4, a spherical electron cloud surrounding an atomic nucleus would best represent: