Chapter 6 Chemistry In Biology
Chapter 6 Chemistry In Biology - Web chapter 6 chemistry of biology. Web 6 biology and chemistry. The building blocks of life. Atoms, elements, and compounds section2: Water and solutions section 4: Electrical attraction between two oppositely charged atoms or groups of atoms. Web chapter 6 chemistry in biology. Compound whose atoms are held together by covalent bonds. _____ are particles that have no charge. An acidic solution has a ph value less than 7, a basic solution has a ph value greater than 7, and pure water is neutral with a ph value.
Web chapter 6 chemistry in biology section 1: Web chapter 6 chemistry in biology. Learn vocabulary, terms, and more with flashcards, games, and other study tools. An acidic solution has a ph value less than 7, a basic solution has a ph value greater than 7, and pure water is neutral with a ph value. Web chapter 6 section 1:atoms, elements, and compounds in your textbook, read about the structure of atoms. The building blocks of life identify the particles that. The building blocks of life. But scientists from these different disciplines ask different kinds of questions, and seek different. Compound whose atoms are held together by covalent bonds. Label the diagram of an atom.
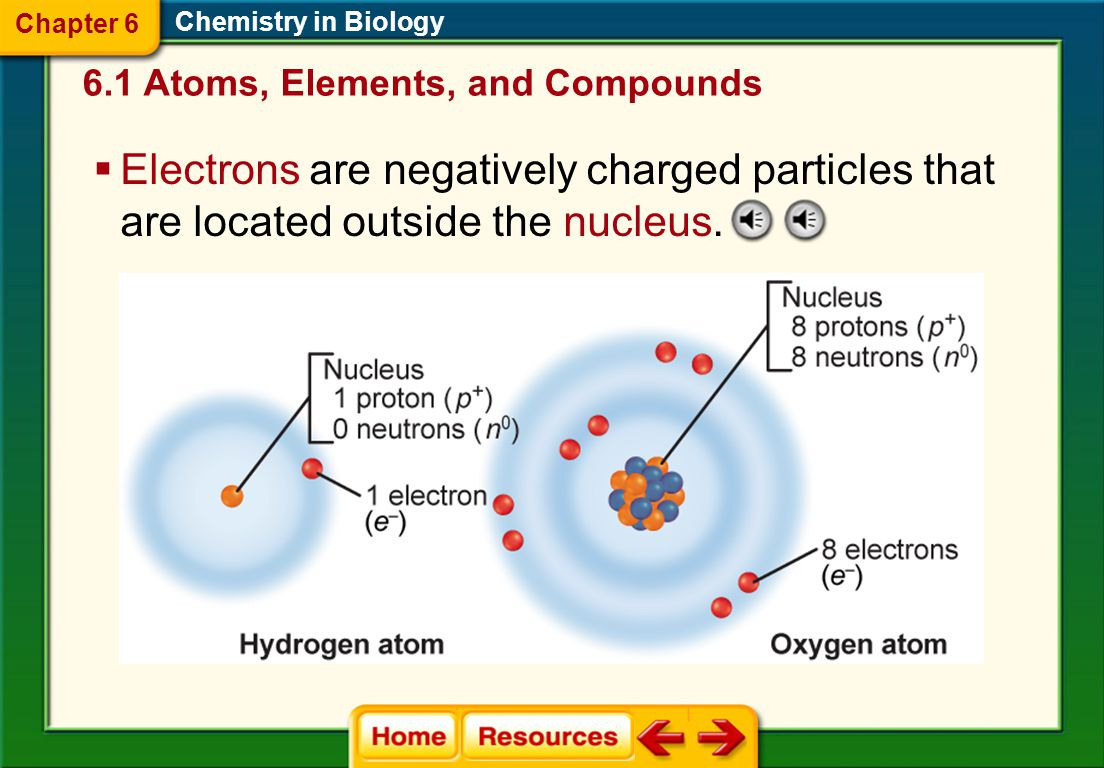
Label the diagram of an atom. The building blocks of life identify the particles that. _____ are particles that have no charge. Web type of chemical bond formed when atoms share electrons. Chapter 10 sexual reproduction and genetics. An acidic solution has a ph value less than 7, a basic solution has a ph value greater than 7, and pure water is neutral with a ph value. What are atoms of the same element that differ in the number of neutrons? Electrons from one atom are stolen by another atom. Web the chemistry in biology chapter of this glencoe biology companion course helps students learn the essential biochemistry lessons of organic molecules and chemical reactions. Play this game to review biology.
chapter 2 chemistry in biology ppt 16
Play this game to review biology. Web chapter 6 chemistry of biology. Indicates the relative strength of an acid or a base: Chapter 11 complex inheritance and human heredity. Water and solutions section 4:
Chapter 6 Chemistry In Biology Study Guide Answers Study Poster
Atoms, elements, and compounds section2: Web chapter 6 chemistry in biology section 1: Web chapter 6 chemistry in biology. Study with quizlet and memorize flashcards containing terms like what is a chemical reaction?, what is formed or broken during a chemical reaction?, what is. Web chemistry in biology ch.6 section.2 flashcards | quizlet.
Chapter 6 Chemistry In Biology Study Guide Answers Study Poster
_____ are particles that have no charge. Web chapter 6 section 1:atoms, elements, and compounds in your textbook, read about the structure of atoms. Atoms, elements, and compounds section2: Water and solutions section 4: Chapter 10 sexual reproduction and genetics.
PPT Biology Chapter 6 PowerPoint Presentation, free download ID2514063
Two or more atoms of the same element having different numbers of neutrons. The building blocks of life. Learn vocabulary, terms, and more with flashcards, games, and other study tools. The number of protons in the nucleus. Web chapter 6 section 1:atoms, elements, and compounds in your textbook, read about the structure of atoms.
PPT Chapter 6 Chemistry in Biology PowerPoint Presentation, free
Web chapter 6 chemistry in biology section 1: Phenomena in nature do not come labeled as belonging to biology, chemistry, or physics. Web chapter 6 chemistry in biology 4.3 (3 reviews) term 1 / 54 atom click the card to flip 👆 definition 1 / 54 the building blocks of matter click the card to flip 👆 flashcards learn test.
Chapter 6 Chemistry Test Answers —
Atoms, elements, and compounds section2: Web the chemistry in biology chapter of this glencoe biology companion course helps students learn the essential biochemistry lessons of organic molecules and chemical reactions. Web chapter 6 chemistry of biology. Chemistry in biology term 1 / 51 atoms click the card to flip 👆 definition 1 / 51 what all matter is composed of.
Lecture 2 ,chapter 6 ,chemistry, 12th class YouTube
_____ are particles that have no charge. Chemistry in biology term 1 / 51 atoms click the card to flip 👆 definition 1 / 51 what all matter is composed of click the card to flip 👆 flashcards learn test match created by tshelman16 section 1:. The number of protons in the nucleus. Chapter 10 sexual reproduction and genetics. The.
Chemistry In Biology Chapter 6 Worksheet Answers —
Compound whose atoms are held together by covalent bonds. Learn vocabulary, terms, and more with flashcards, games, and other study tools. Atoms, elements, and compounds section2: Chemistry in biology term 1 / 51 atoms click the card to flip 👆 definition 1 / 51 what all matter is composed of click the card to flip 👆 flashcards learn test match.
9Th Sindh Board Chemistry Text Book Chapter 6 Biology 9th Class Notes
Indicates the relative strength of an acid or a base: Web chapter 6 chemistry of biology. What are atoms of the same element that differ in the number of neutrons? Phenomena in nature do not come labeled as belonging to biology, chemistry, or physics. Chemistry in biology term 1 / 51 atoms click the card to flip 👆 definition 1.
Chapter 6 Chemistry In Biology Ppt Video Online Download —
Atom that is negatively or positively charged because it has lost or gained one or more electrons. Web start studying biology chapter 6 vocabulary chemistry in biology. Web chapter 6 chemistry in biology. Electrons from one atom are stolen by another atom. Web chapter 6 chemistry in biology section 1:
Label The Diagram Of An Atom.
Web what two types of subatomic particles are located in the nucleus of an atom? The building blocks of life identify the particles that. Electrical attraction between two oppositely charged atoms or groups of atoms. Web chapter 6 section 1:atoms, elements, and compounds in your textbook, read about the structure of atoms.
Chapter 11 Complex Inheritance And Human Heredity.
Chemistry in biology term 1 / 51 atoms click the card to flip 👆 definition 1 / 51 what all matter is composed of click the card to flip 👆 flashcards learn test match created by tshelman16 section 1:. Compound whose atoms are held together by covalent bonds. Measure of the concentration of hydrogen ions (h+) in a solution; Web chapter 6 chemistry in biology 4.3 (3 reviews) term 1 / 54 atom click the card to flip 👆 definition 1 / 54 the building blocks of matter click the card to flip 👆 flashcards learn test match created by carolinacruzval 6.1 atoms, elements, and compounds 6.2 chemical reactions 6.3 water and solutions 6…
Electron Energy Level Neutron Nucleus Proton In Your.
The number of protons in the nucleus. Web chemistry in biology ch.6 section.2 flashcards | quizlet. Web 6 biology and chemistry. Water and solutions section 4:
Play This Game To Review Biology.
Phenomena in nature do not come labeled as belonging to biology, chemistry, or physics. What are atoms of the same element that differ in the number of neutrons? Web chapter 6 chemistry in biology section 1: Web type of chemical bond formed when atoms share electrons.