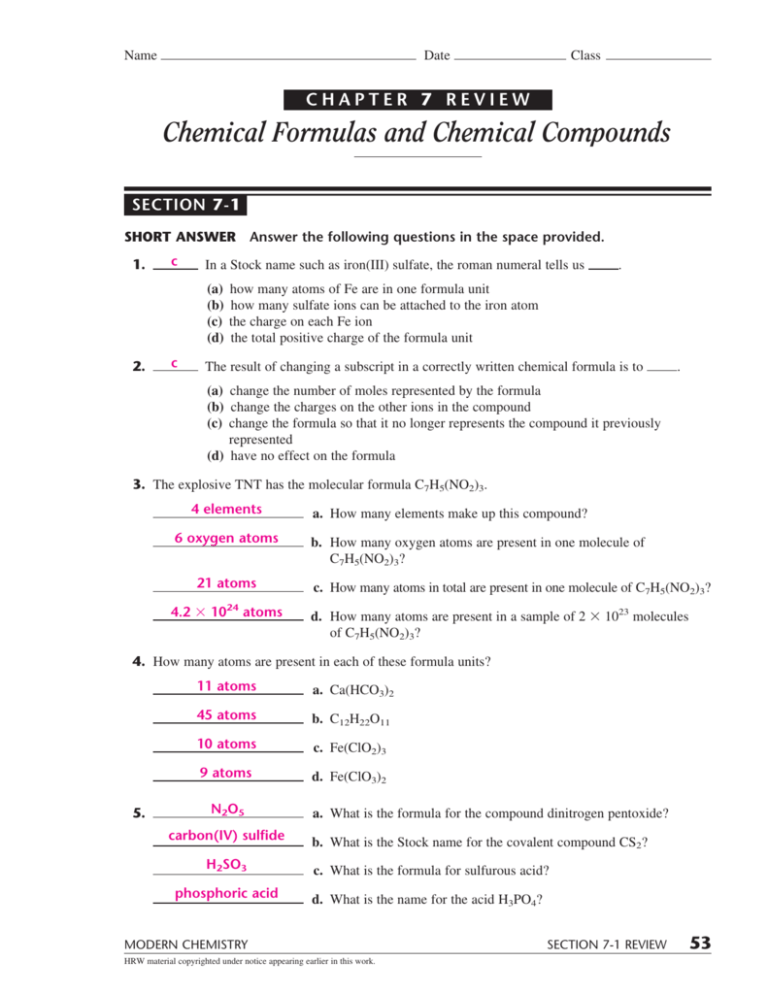
Chapter 7 Review Chemical Formulas And Chemical Compounds
Chapter 7 Review Chemical Formulas And Chemical Compounds - (b) how many sulfate ions can be attached to the iron atom. Web benzene, chemical compound, carbonyl compounds, carboxylic acids, acyl compounds, chemical bonding, chemistry of life, electrode potential, electrons in atoms, enthalpy change, equilibrium, group iv, groups ii and vii, halogenoalkanes, hydrocarbons, introduction to organic chemistry… Atomic mass of each element. (c) the charge on each fe ion. Number of atoms of each element in one molecule of a compound c. Some of the group 2 metals react with oxygen to form oxides. Web chapter 7 review : Chemical formula writing worksheet review with answer key.pdf. What is the empirical formula for a compound. Click the card to flip 👆 a chemical formula includes the symbols of the elements in the compound.
Chemical formulas and chemical compounds (mixed review) copper (ii) carbonate click the card to flip 👆 cuco₃ click the card to flip 👆 1 / 28 flashcards test created by blonde10123 terms in this set. _____ in a stock system name such as iron(iii) sulfate, the roman numeral tells us (a) how many atoms of fe are in one formula unit. (d) the total positive charge of the formula unit. Some of the group 2 metals react with oxygen to form oxides. In a stock system name such as iron(iii) sulfate, the roman numeral tells us (a) how many atoms of fe are in one formula. A chemical formula includes the symbols of the elements in the compound and subscripts that indicate. Click the card to flip 👆 a chemical formula includes the symbols of the elements in the compound. Number of atoms of each element in one molecule of a compound c. (b) how many sulfate ions can be attached to the iron atom. Naming system or binary ionic compounds involves combining names of compounds.
Common chemicals and their common names. (b) how many sulfate ions can be attached to the iron atom. C) type of bond holding particles together in a compound. Some of the group 2 metals react with oxygen to form peroxides. Chapter 5 the legislative branch 41 terms. Web review the common reactions of group 2 metals in the elements handbook (appendix a), and answer the following questions: D) distribution of electrons among the bonded particles in a compound. Web modern chemistry 61 chemical bonding chapter 7 review chemical formulas and chemical compounds mixed review short answer answer the following questions in the space provided. (d) the total positive charge of the formula unit. The atoms in a pure.
PPT Chapter 7 Chemical Formulas & Compounds PowerPoint Presentation
Some of the group 2 metals react with oxygen to form oxides. Chemical compounds and chemical formulas. Valence electrons and oxidation state. The oxidation numbers of all the elements in the compound d. Chemical formula writing worksheet review with answer key.pdf.
Chemical Compounds 1er Edition 1. 3 Volume in pdf Science
Total number of positive and negative charges must be equal. Naming ionic and covalent compounds. Naming system or binary ionic compounds involves combining names of compounds. Web modern chemistry 61 chemical bonding chapter 7 review chemical formulas and chemical compounds mixed review short answer answer the following questions in the space provided. The formula mass of the compound c.
Chapter 7 Chemical Formulas And Chemical Compounds LarsDamians
Web a) charge on an ion. The formula mass of the compound c. Significance of a chemical formula a. Number of atoms or ions of each element that are combined in the compound. Web answer the following questions in the space provided.
Chapter 7 Chemical Formulas and Chemical Compounds
Click the card to flip 👆. Write formulas for the following compounds… Valence electrons and oxidation state. The number of moles of the compound b. Some of the group 2 metals react with oxygen to form oxides.
Chemical Compounds, their Common Names, Formulas & Uses Download PDF
What is the empirical formula for a compound. The atoms in a pure. Web chapter 7 review : Click the card to flip 👆. _____ changing a subscript in a correctly written chemical formula
Chapter 7 Chemical Formulas And Chemical Compounds LarsDamians
C) type of bond holding particles together in a compound. The atoms in a pure. Ionic compounds are composed to elements in an _______________ bond. Number of atoms of each element in one molecule of a compound c. Web chapter 7 review :
PPT Modern Chemistry Chapter 7 Chemical Formulas & Chemical Compounds
Number of atoms or ions of each element that are combined in the compound. The oxidation numbers of all the elements in the compound d. Web a) charge on an ion. Naming ionic and covalent compounds. Other sets by this creator.
10+ Chapter 7 Review Chemical Formulas And Chemical Compounds Answer
Web chemistry chapter 7 review (names and formulas) term. Click the card to flip 👆. (c) the charge on each fe ion. Write the formulas for these compounds. Web a) charge on an ion.
CHEMISTRY at Crossroads Middle School StudyBlue
Naming system or binary ionic compounds involves combining names of compounds. Total number of positive and negative charges must be equal. Chemical compounds and chemical formulas. Web chapter 7 review : Web chapter 7 review chemical formulas and chemical compounds section 1 short answer answer the following questions in the space provided.
Compounds Chemical Formulae Teaching Resources
6= ethane (2 carbon atoms, 6 hydrogen atoms) b. Number of atoms or ions of each element that are combined in the compound. The number of moles of the compound b. Chemical formula writing worksheet review with answer key.pdf. Web chemistry chapter 7 review (names and formulas) term.
Write Formulas For The Following Compounds…
(d) the total positive charge of the formula unit. Valence electrons and oxidation state. Web benzene, chemical compound, carbonyl compounds, carboxylic acids, acyl compounds, chemical bonding, chemistry of life, electrode potential, electrons in atoms, enthalpy change, equilibrium, group iv, groups ii and vii, halogenoalkanes, hydrocarbons, introduction to organic chemistry… Some of the group 2 metals react with oxygen to form oxides.
D) Distribution Of Electrons Among The Bonded Particles In A Compound.
Oxidation numbers are assigned to the atoms composing a compound or ion in order to indicate the general distribution of electrons among the bonded atoms in the compound or ion. 6= ethane (2 carbon atoms, 6 hydrogen atoms) b. Web modern chemistry 61 chemical bonding chapter 7 review chemical formulas and chemical compounds mixed review short answer answer the following questions in the space provided. Web chapter 7 review :
A Chemical Formula Includes The Symbols Of The Elements In The Compound And Subscripts That Indicate.
C) type of bond holding particles together in a compound. A chemical formula for a molecular compound represents the composition of. The oxidation numbers of all the elements in the compound d. Covalent compounds naming and review.
Chapter 5 The Legislative Branch 41 Terms.
Click the card to flip 👆. Atomic mass of each element. Click the card to flip 👆. What is true about the charges in a binary compound.