Chapter 8 Review Chemistry
Chapter 8 Review Chemistry - Web terms in this set (44) a diatomic molecule contains two or three atoms. Strengths of covalent bonds bond order is the number of electron pairs that hold two atoms together. What does it mean to say an equation is balanced? Web chapter 8 review chemical equations and reactions section 1 short answer answer the following questions in the space provided. Web chemistry chapter 8 review 4.3 (3 reviews) get a hint explain why elements such as p, n, and s have positive oxidation numbers in some compounds but have negative oxidation numbers in others? The following pages contain the bulk (but not all) of the information for the chapter. Web chemistry chapter 8 review questions list the four observations that indicate that a chemical reaction may have taken place click the card to flip 👆 chemical produced energy change (light or heat) formation of. Why is it important for an equation to be balanced? Web we go over hydroboration, markovnikov additions, kmno4 and oso4 reactions, and a ton of other reaction mechanisms. Web unit 6 more about chemical reactions.
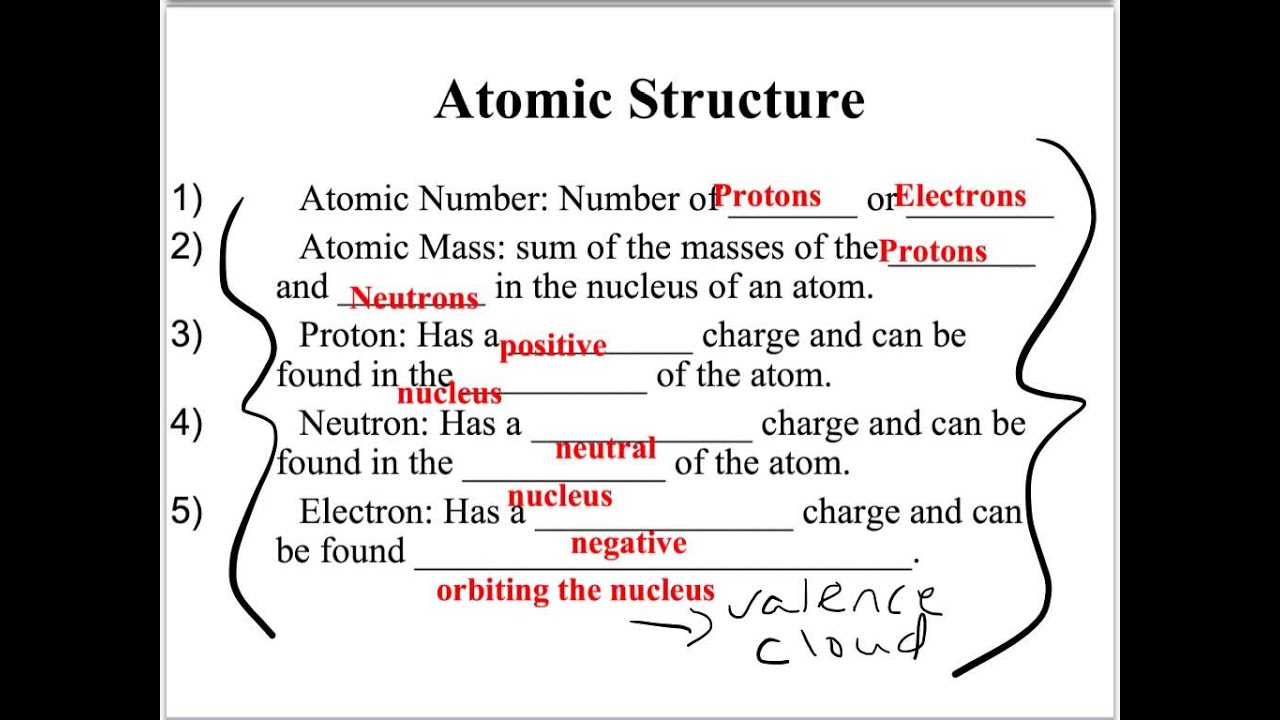
They form crystal lattices because their positive ions and negative ions are so tightly attracted. Web determining reaction types and solubility rules note sheets.pdf. 1.match the symbol on the left with its appropriate description on the right. Web terms in this set (44) a diatomic molecule contains two or three atoms. Write the balanced chemical equation for the combustion of c2h2 in oxygen. Web bju chemistry chapter 8 review 2.8 (4 reviews) oxidation number (on) click the card to flip 👆 the number of electrons (the charge) that an atom in a compound must gain or lose to return to its neutral state;. Click the card to flip 👆 döbereiner one of the ealiest chemists to show similarity of properties for groups of elements by arranging them in. An introduction to organic synthesis alkynes contain triple bond acetylene: Contains protons, neutrons, and electrons. The central core of an atom containing protons and neutrons.
Web unit 6 more about chemical reactions. Both types of bonds result from overlap of atomic orbitals on adjacent atoms and contain a maximum of two electrons. They form crystal lattices because their positive ions and negative ions are so tightly attracted. Web terms in this set (44) a diatomic molecule contains two or three atoms. Unit 10 gases and kinetic molecular theory. Web terms in this set (20) why do ionic compounds form crystal lattices? Web determining reaction types and solubility rules note sheets.pdf. Write the balanced chemical equation for the combustion of c2h2 in oxygen. Why is it important for an equation to be balanced? Web chemistry chapter 8 review questions list the four observations that indicate that a chemical reaction may have taken place click the card to flip 👆 chemical produced energy change (light or heat) formation of.
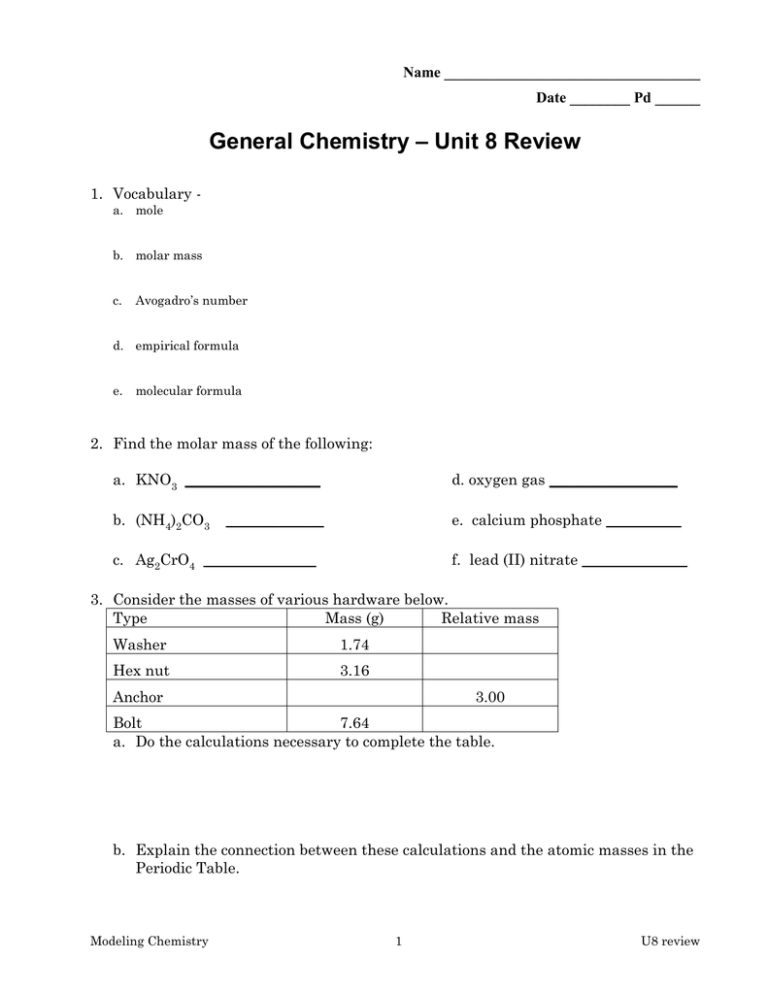
Unit 8 Review General Chemistry
What is an ionic compound whose solution conducts an electric current? Smallest particle of an element; 2c2h2(g) 5o2(g) 4co2(g) 2h2o(l ) → 2.0 mol if 1.0. Unit 7 electronic structure of atoms. Web chemistry chapter 8 review questions list the four observations that indicate that a chemical reaction may have taken place click the card to flip 👆 chemical produced.
AP Chemistry Chapter 8 & 9 Practice Test with answers (1)
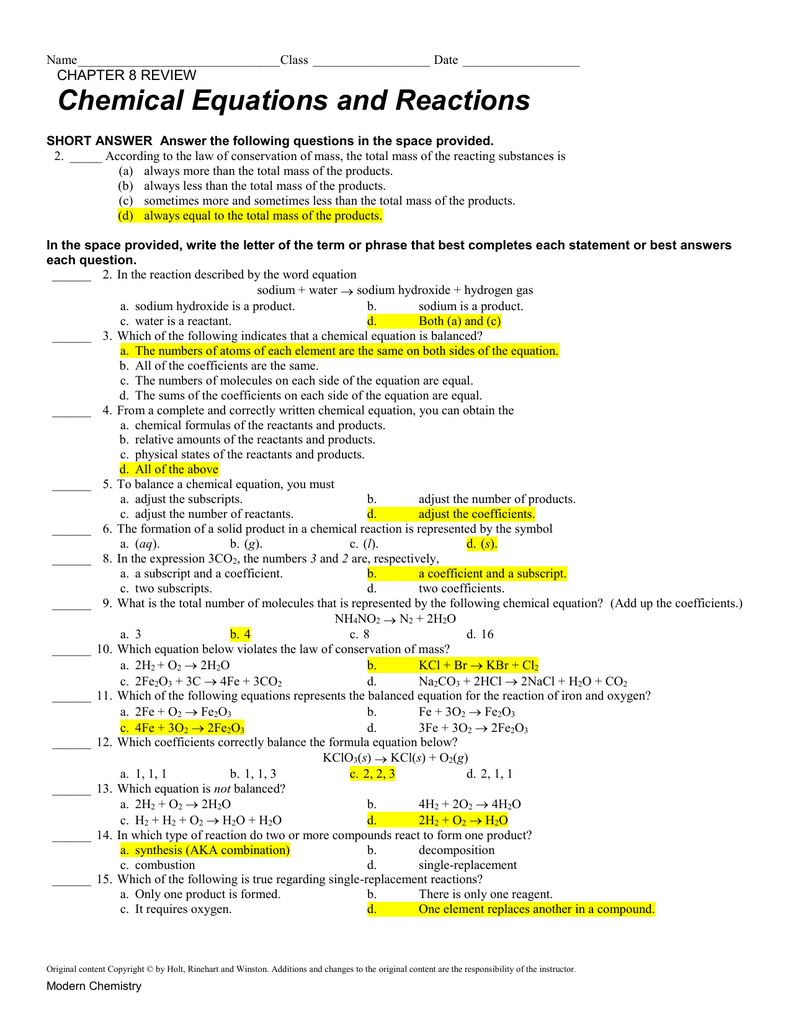
Web modern chemistry 1 chemical equations and reactions chapter 8 review chemical equations and reactions teacher notes and answers chapter 8 section 1 short answer 1. They form crystal lattices because their positive ions and negative ions are so tightly attracted. Web we go over hydroboration, markovnikov additions, kmno4 and oso4 reactions, and a ton of other reaction mechanisms. 1.match.
Spice of Lyfe Chemical Equations And Reactions Chapter 8 Review Answers
Web we go over hydroboration, markovnikov additions, kmno4 and oso4 reactions, and a ton of other reaction mechanisms. Web determining reaction types and solubility rules note sheets.pdf. Web terms in this set (20) why do ionic compounds form crystal lattices? Acetylene gas, c2h2, is burned to provide the high temperature needed in welding. Web chapter 8 chemistry review the state.
Fantastic Chapter 8 Chemistry Test Answers Chemical Equations And
Smallest particle of an element; Why is it important for an equation to be balanced? Both types of bonds result from overlap of atomic orbitals on adjacent atoms and contain a maximum of two electrons. Unit 11 states of matter and intermolecular forces. 2c2h2(g) 5o2(g) 4co2(g) 2h2o(l ) → 2.0 mol if 1.0.
Spice of Lyfe Chemical Equations And Reactions Chapter 8 Review Answers
The molecular structure of carbon dioxide is one carbon. Unit 7 electronic structure of atoms. Web chapter 8 chemistry review the state of matter (phase) for a reactant or a product in a chemical equation is indicated by a. Web bju chemistry chapter 8 review 2.8 (4 reviews) oxidation number (on) click the card to flip 👆 the number of.
Chemistry Chapter 8 Review Problems YouTube
Web we go over hydroboration, markovnikov additions, kmno4 and oso4 reactions, and a ton of other reaction mechanisms. Click the card to flip 👆. Acetylene gas, c2h2, is burned to provide the high temperature needed in welding. Why is it important for an equation to be balanced? They form crystal lattices because their positive ions and negative ions are so.
Selina Concise Chemistry Class 8 ICSE Solutions Chapter 1 Matter
Unit 7 electronic structure of atoms. Contains protons, neutrons, and electrons. What does it mean to say an equation is balanced? Web review of important concepts. 2c2h2(g) 5o2(g) 4co2(g) 2h2o(l ) → 2.0 mol if 1.0.
Chapter 8 Practice Test Doral Academy Preparatory
Both types of bonds result from overlap of atomic orbitals on adjacent atoms and contain a maximum of two electrons. Web chemistry chapter 8 review questions list the four observations that indicate that a chemical reaction may have taken place click the card to flip 👆 chemical produced energy change (light or heat) formation of. An introduction to organic synthesis.
Chemistry Final Review YouTube
Are ionic bonds chemical or physical reactions? What is an ionic compound whose solution conducts an electric current? Web unit 6 more about chemical reactions. Web terms in this set (20) why do ionic compounds form crystal lattices? Web determining reaction types and solubility rules note sheets.pdf.
CBSE 12th Chemistry Board Exam 2020 Important Questions & Answers from
Web 4.4 (5 reviews) tell the contributions of döbereiner to the periodic table. Unit 11 states of matter and intermolecular forces. The molecular structure of carbon dioxide is one carbon. Web holt modern chemistry review. Is the common name for ethyne, used
They Form Crystal Lattices Because Their Positive Ions And Negative Ions Are So Tightly Attracted.
1.match the symbol on the left with its appropriate description on the right. Why is it important for an equation to be balanced? Web bju chemistry chapter 8 review 2.8 (4 reviews) oxidation number (on) click the card to flip 👆 the number of electrons (the charge) that an atom in a compound must gain or lose to return to its neutral state;. Unit 7 electronic structure of atoms.
Both Types Of Bonds Result From Overlap Of Atomic Orbitals On Adjacent Atoms And Contain A Maximum Of Two Electrons.
Are ionic bonds chemical or physical reactions? Unit 11 states of matter and intermolecular forces. Web holt modern chemistry review. Strengths of covalent bonds bond order is the number of electron pairs that hold two atoms together.
Web Unit 6 More About Chemical Reactions.
Web terms in this set (20) why do ionic compounds form crystal lattices? Web chemistry chapter 8 review 4.3 (3 reviews) get a hint explain why elements such as p, n, and s have positive oxidation numbers in some compounds but have negative oxidation numbers in others? Single bonds have a bond order of one, and multiple bonds with bond orders of two (a double bond) and three (a. Unit 10 gases and kinetic molecular theory.
Click The Card To Flip 👆.
Web chapter 8 chemistry review the state of matter (phase) for a reactant or a product in a chemical equation is indicated by a. Write the balanced chemical equation for the combustion of c2h2 in oxygen. What is an ionic compound whose solution conducts an electric current? Web 4.4 (5 reviews) tell the contributions of döbereiner to the periodic table.