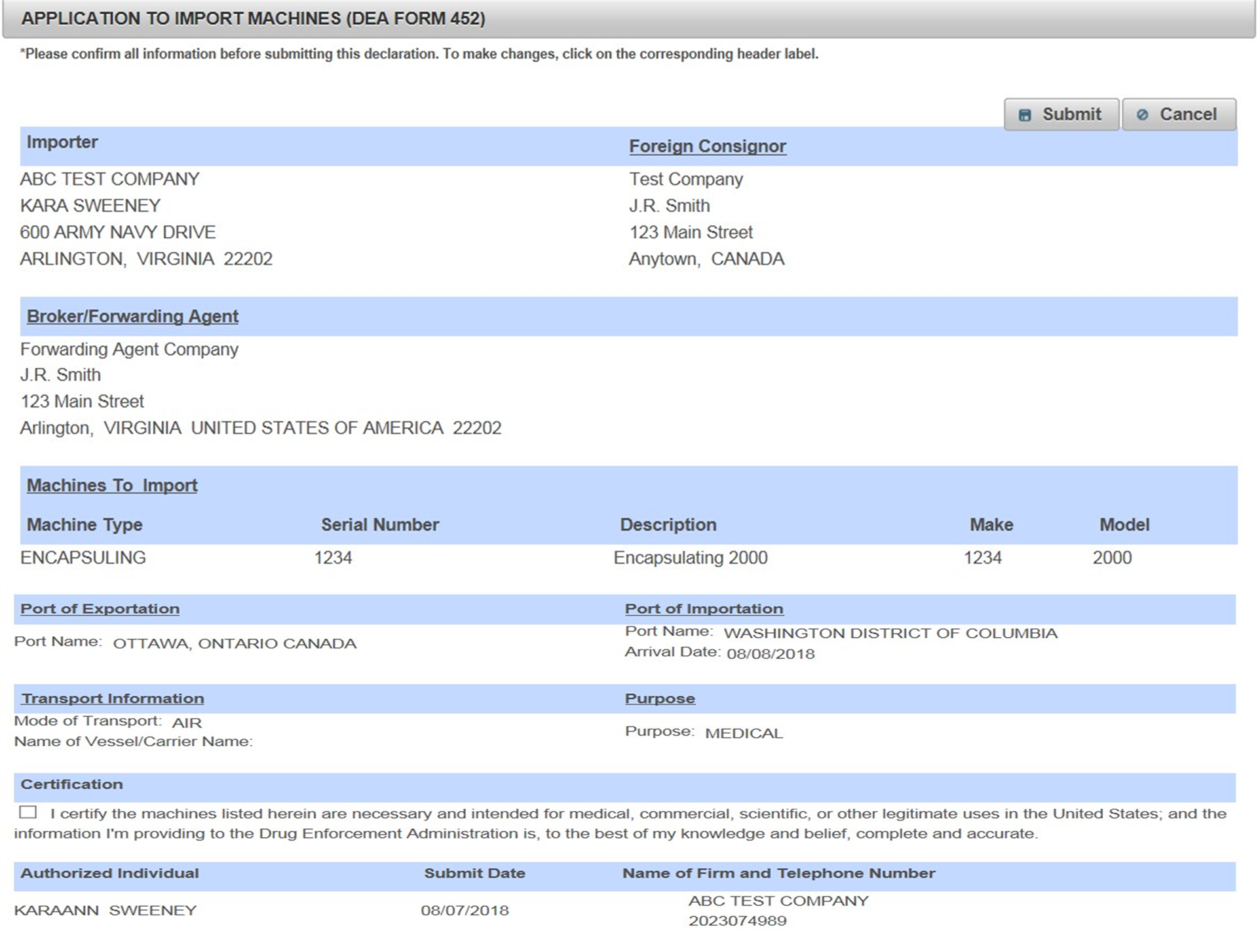
Dea Form 41 Is Used To
Dea Form 41 Is Used To - Name of drug or preparation registrants will fill in columns 1,2,3, and 4 only. Web the dea form 41 shall list the controlled substance or substances which the registrant desires to dispose. Web created by asoto1_uisd_net teacher terms in this set (12) dea form 41 used to return or destroy drugs per dea approval must be submitted at least 2 weeks in advance dea. Are to be destroyed by: ( ii) the special agent in charge shall instruct the registrant to. Web a dea form 41 must be used when destroying expired or unwanted controlled substances. The only time university researchers use form 41 is after controlled. Web the dea form 41 shall list the controlled substance or substances which the registrant desires to dispose. The dea is modifying the form 41 to be the single document used by both closed systems (e.g., health systems) as well as by reverse. Web in addition, the dea has modified dea form 41 and is explicitly requiring that form to be used to record the destruction of controlled substances that remain in the.
Web the dea form 41 shall list the controlled substance or substances which the registrant desires to dispose. Web the dea form 41 shall list the controlled substance or substances which the registrant desires to dispose. The dea form 41 is available online here. Web dea form 41 is used to request permission from the dea to destroy controlled substances. Web when do i have to use a dea form 41? ( ii) the special agent in charge shall instruct the registrant to. Web when do i have to use a dea form 41? Web the revised form is entitled “registrant record of controlled substances destroyed, dea form 41.” the existing dea form 41, which expires on august 31, 2014, and was. Web created by asoto1_uisd_net teacher terms in this set (12) dea form 41 used to return or destroy drugs per dea approval must be submitted at least 2 weeks in advance dea. It is recommended that registrants review 21 cfr §1317 — disposal.
The only time university researchers use form 41 is after controlled. The dea is modifying the form 41 to be the single document used by both closed systems (e.g., health systems) as well as by reverse. Web the revised form is entitled “registrant record of controlled substances destroyed, dea form 41.” the existing dea form 41, which expires on august 31, 2014, and was. ( ii) the special agent in charge shall instruct the registrant to. Web complete dea form 41 (registrant’s inventory of drugs surrendered) write a letter to the dea to request permission to destroy, including: Web when do i have to use a dea form 41? Web dea defines institutional practitioner to mean a hospital or other person (other than an individual) licensed, registered, or otherwise permitted by the united. It is recommended that registrants review 21 cfr §1317 — disposal. Web in addition, the dea has modified dea form 41 and is explicitly requiring that form to be used to record the destruction of controlled substances that remain in the. Are to be destroyed by:
Registrant Disclosure of Loss Diversion or Destruction of Controlled
Web the revised form is entitled “registrant record of controlled substances destroyed, dea form 41.” the existing dea form 41, which expires on august 31, 2014, and was. Web the dea form 41 shall list the controlled substance or substances which the registrant desires to dispose. The dea form 41 is available online here. Web please complete the dea form.
Medical Exporters And Importers Usa Mail Documents Required For
The dea is modifying the form 41 to be the single document used by both closed systems (e.g., health systems) as well as by reverse. Web the dea form 41 shall list the controlled substance or substances which the registrant desires to dispose. It is recommended that registrants review 21 cfr §1317 — disposal. Web complete dea form 41 (registrant’s.
DEA Form 357 Download Fillable PDF or Fill Online Application for
Name of drug or preparation registrants will fill in columns 1,2,3, and 4 only. Web a dea form 41 must be used when destroying expired or unwanted controlled substances. Web the revised form is entitled “registrant record of controlled substances destroyed, dea form 41.” the existing dea form 41, which expires on august 31, 2014, and was. Web complete dea.
Most frequent keywords of DEArelated publication Download Scientific
The dea is modifying the form 41 to be the single document used by both closed systems (e.g., health systems) as well as by reverse. Name of drug or preparation registrants will fill in columns 1,2,3, and 4 only. ( ii) the special agent in charge shall instruct the registrant to. Web the dea form 41 shall list the controlled.
Controlled Substances Compliance for Transport Programs Air Medical
Web the revised form is entitled “registrant record of controlled substances destroyed, dea form 41.” the existing dea form 41, which expires on august 31, 2014, and was. ( ii) the special agent in charge shall instruct the registrant to. ( ii) the special agent in charge shall instruct the registrant to. The dea form 41 is available online here..
To Apply for DEA License in 10 minutes IPharmachine
( ii) the special agent in charge shall instruct the registrant to. Web complete dea form 41 (registrant’s inventory of drugs surrendered) write a letter to the dea to request permission to destroy, including: Web the dea form 41 shall list the controlled substance or substances which the registrant desires to dispose. Name of drug or preparation registrants will fill.
File S Bridgerecovery Nsg Folder Med Destruction Controlled Medication
( ii) the special agent in charge shall instruct the registrant to. The dea is modifying the form 41 to be the single document used by both closed systems (e.g., health systems) as well as by reverse. Web created by asoto1_uisd_net teacher terms in this set (12) dea form 41 used to return or destroy drugs per dea approval must.
Dea Application Form Fill Out and Sign Printable PDF Template signNow
It is recommended that registrants review 21 cfr §1317 — disposal. Web the revised form is entitled “registrant record of controlled substances destroyed, dea form 41.” the existing dea form 41, which expires on august 31, 2014, and was. Web when do i have to use a dea form 41? ( ii) the special agent in charge shall instruct the.
Top 6 Dea Form 41 Templates free to download in PDF format
It is recommended that registrants review 21 cfr §1317 — disposal. Web a dea form 41 must be used when destroying expired or unwanted controlled substances. Web in addition, the dea has modified dea form 41 and is explicitly requiring that form to be used to record the destruction of controlled substances that remain in the. Web the dea form.
Where Do You Mail Dea 222 Forms Fill Online, Printable, Fillable
Web the revised form is entitled “registrant record of controlled substances destroyed, dea form 41.” the existing dea form 41, which expires on august 31, 2014, and was. ( ii) the special agent in charge shall instruct the registrant to. The dea form 41 is available online here. Web the dea form 41 shall list the controlled substance or substances.
The Dea Is Modifying The Form 41 To Be The Single Document Used By Both Closed Systems (E.g., Health Systems) As Well As By Reverse.
Web complete dea form 41 (registrant’s inventory of drugs surrendered) write a letter to the dea to request permission to destroy, including: Web when do i have to use a dea form 41? The dea form 41 is available online here. Web the revised form is entitled “registrant record of controlled substances destroyed, dea form 41.” the existing dea form 41, which expires on august 31, 2014, and was.
Web When Do I Have To Use A Dea Form 41?
The dea is modifying the form 41 to be the single document used by both closed systems (e.g., health systems) as well as by reverse. The only time university researchers use form 41 is after controlled. ( ii) the special agent in charge shall instruct the registrant to. Name of drug or preparation registrants will fill in columns 1,2,3, and 4 only.
Web Created By Asoto1_Uisd_Net Teacher Terms In This Set (12) Dea Form 41 Used To Return Or Destroy Drugs Per Dea Approval Must Be Submitted At Least 2 Weeks In Advance Dea.
It is recommended that registrants review 21 cfr §1317 — disposal. Web please complete the dea form 41 and submit to ehs to request disposal of dea controlled substances. Are to be destroyed by: Web in addition, the dea has modified dea form 41 and is explicitly requiring that form to be used to record the destruction of controlled substances that remain in the.
Web Dea Form 41 Is Used To Request Permission From The Dea To Destroy Controlled Substances.
Web the dea form 41 shall list the controlled substance or substances which the registrant desires to dispose. Web the dea form 41 shall list the controlled substance or substances which the registrant desires to dispose. Web dea defines institutional practitioner to mean a hospital or other person (other than an individual) licensed, registered, or otherwise permitted by the united. ( ii) the special agent in charge shall instruct the registrant to.