Do Metals Form Cations
Do Metals Form Cations - Closely packed cations and loosely held valence electrons rather than neutral atoms what can the valence electrons in a pure metal be modeled as? Web what do metals consist of? All chemical reactions are driven to gain more. Web cations are atoms that contain a positive charge, and they are formed when the atoms lose electrons which are negatively charged. Oxygen, nitrogen, sulfur), while most metals form cations (e.g. Some examples of cations are calcium (ca 2+ ), potassium (k + ), hydrogen (h + ). Writing chemical formulas when writing the formula of a compound, the cation is listed before the anion. Cl atoms gain 1 electron, therefore making them anions. Pearson higher level chemistry textbook, 2nd edition. By catrin brown and mike ford.
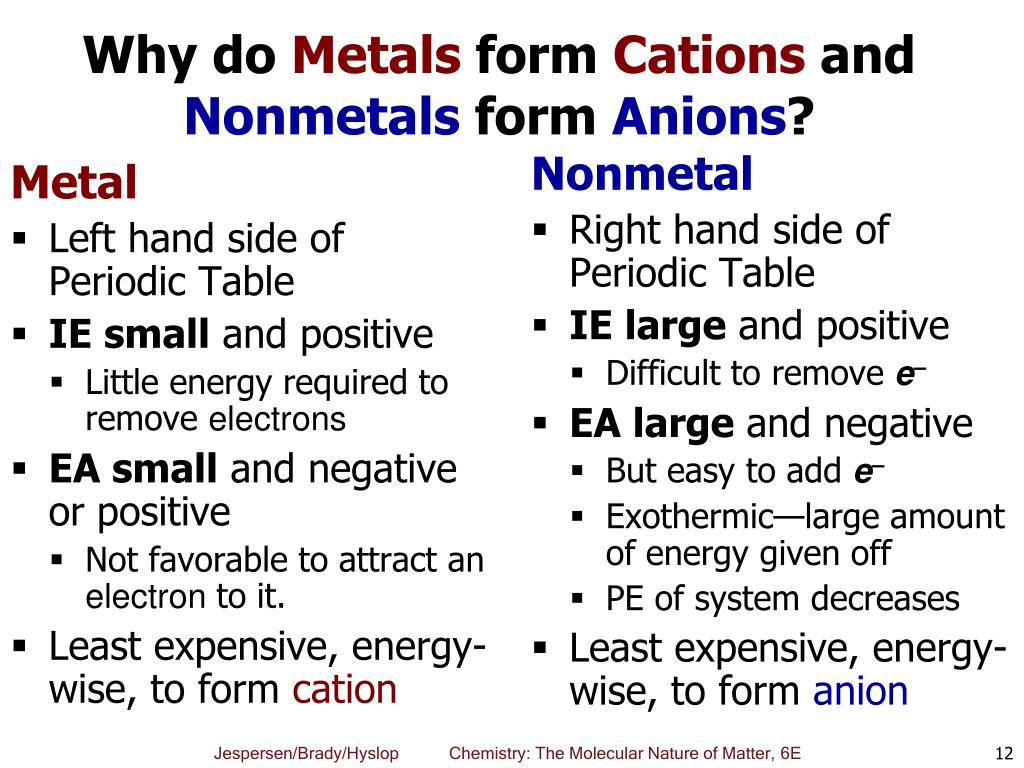
Oxygen, nitrogen, sulfur), while most metals form cations (e.g. Web having lost electrons, which are negatively charged, atoms of metals therefore gain a net positive charge i.e they become cations. By catrin brown and mike ford. Some examples of cations are calcium (ca 2+ ), potassium (k + ), hydrogen (h + ). Web cations are positively charged ions. Closely packed cations and loosely held valence electrons rather than neutral atoms what can the valence electrons in a pure metal be modeled as? The solvation number , n , determined by a variety of experimental methods is 4 for li + and be 2+ and 6 for most elements in periods 3. This is the typical behavior for many metal substances. They lose one or more than one electron. Web metals tend to form cations while nonmetals tend to form anions.
They are formed when a metal loses its electrons. Closely packed cations and loosely held valence electrons rather than neutral atoms what can the valence electrons in a pure metal be modeled as? Transition metals often form ions wihout complete octets that's why all the stable ions are all cations. Therefore, they possess a net positive charge. Writing chemical formulas when writing the formula of a compound, the cation is listed before the anion. And because of this behavior,. Web what do metals consist of? Web having lost electrons, which are negatively charged, atoms of metals therefore gain a net positive charge i.e they become cations. Web cations are atoms that contain a positive charge, and they are formed when the atoms lose electrons which are negatively charged. Cl atoms gain 1 electron, therefore making them anions.
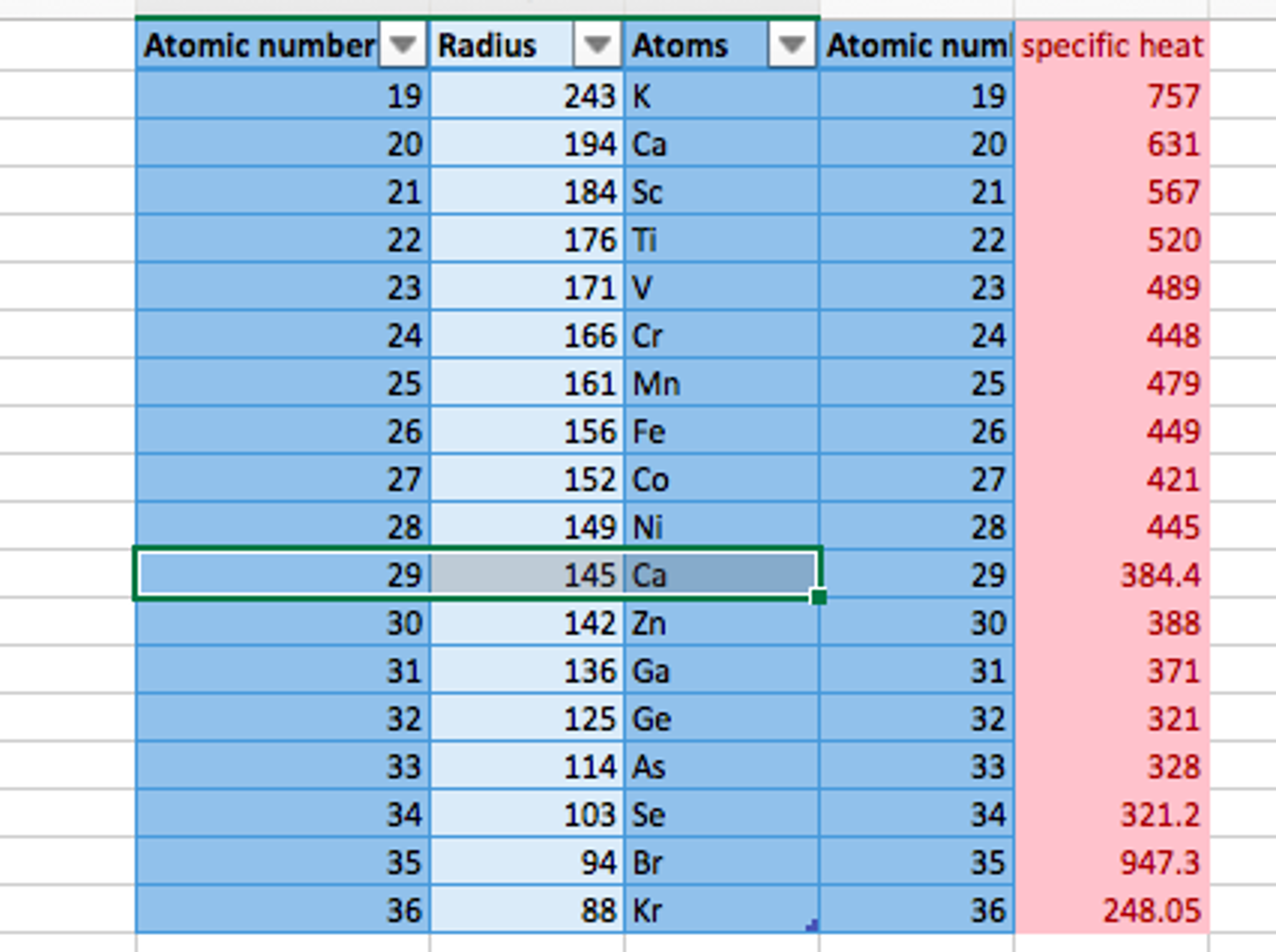
Form a table of alkaline earth metal cations and the
All chemical reactions are driven to gain more. Web halogens always form anions, alkali metals and alkaline earth metals always form cations. Web a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m(h 2 o) n] z+. Transition metals often form ions wihout complete octets that's why all the stable ions.
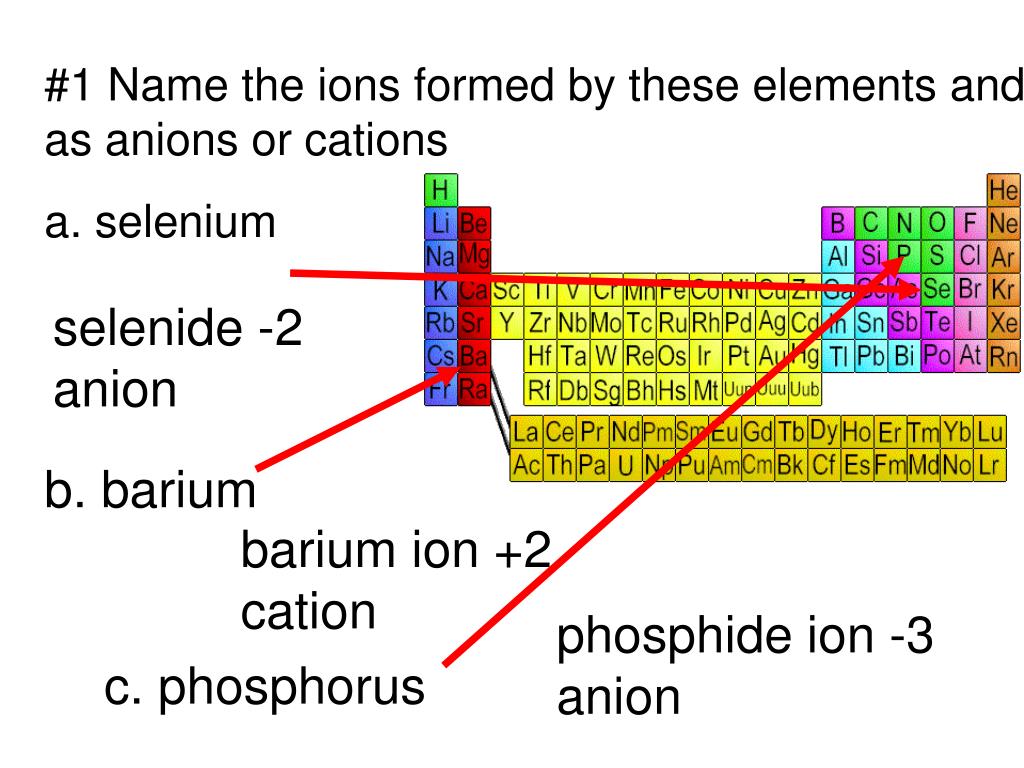
PPT 1 Name the ions formed by these elements and classify them as
All chemical reactions are driven to gain more. Web what do metals consist of? Web metals tend to form cations while nonmetals tend to form anions. Writing chemical formulas when writing the formula of a compound, the cation is listed before the anion. Web alkali metals and alkaline earth metals always form cations.
*2.7 Nomenclature Chemistry LibreTexts
Therefore, they possess a net positive charge. Web a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m(h 2 o) n] z+. It has fewer electrons than protons. Transition metals often form ions wihout complete octets that's why all the stable ions are all cations. Closely packed cations and loosely held.
Do metals form anions or cations quizlet? Book Vea
Oxygen, nitrogen, sulfur), while most metals form cations (e.g. Some examples of cations are calcium (ca 2+ ), potassium (k + ), hydrogen (h + ). Most other nonmetals typically form anions (e.g. Web alkali metals and alkaline earth metals always form cations. Web metals tend to form cations while nonmetals tend to form anions.
is incorreer? all s area eleseis erand e are metals b, all p area
Web a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m(h 2 o) n] z+. Some examples of cations are calcium (ca 2+ ), potassium (k + ), hydrogen (h + ). You can also tell that they form cations because some. Writing chemical formulas when writing the formula of a.
PPT Chapter 9 The Basics of Chemical Bonding PowerPoint Presentation
This is the typical behavior for many metal substances. They are formed when a metal loses its electrons. Transition metals often form ions wihout complete octets that's why all the stable ions are all cations. Pearson higher level chemistry textbook, 2nd edition. Web cations are atoms that contain a positive charge, and they are formed when the atoms lose electrons.
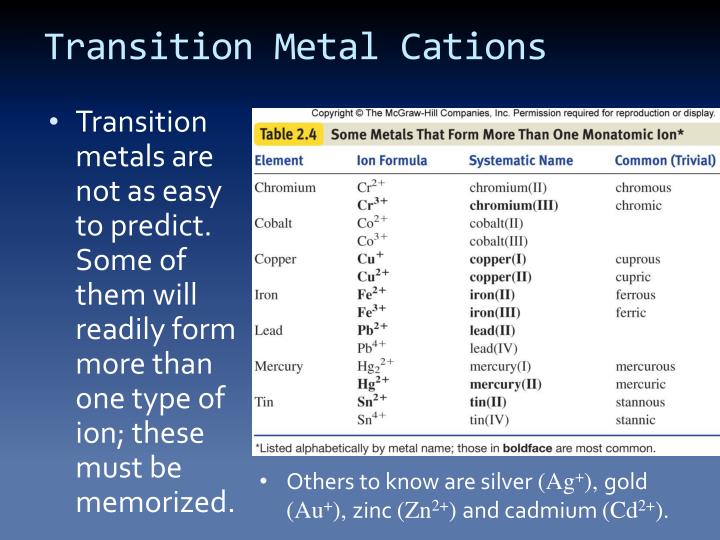
Solved Question 6 (1 point) Why do metals tend to form
What types of ions do the metallic and the nonmetallic elements form? Cl atoms gain 1 electron, therefore making them anions. The solvation number , n , determined by a variety of experimental methods is 4 for li + and be 2+ and 6 for most elements in periods 3. Therefore, they possess a net positive charge. Pearson higher level.
PPT IONS PowerPoint Presentation ID2435906
Web cations are atoms that contain a positive charge, and they are formed when the atoms lose electrons which are negatively charged. Some examples of cations are calcium (ca 2+ ), potassium (k + ), hydrogen (h + ). And because of this behavior,. Transition metals often form ions wihout complete octets that's why all the stable ions are all.
Chem matters ch6_ionic_bond
Web a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m(h 2 o) n] z+. Web halogens always form anions, alkali metals and alkaline earth metals always form cations. This is the typical behavior for many metal substances. Some examples of cations are calcium (ca 2+ ), potassium (k + ),.
PPT Naming IONS & formulas for Ionic Compounds PowerPoint
What types of ions do the metallic and the nonmetallic elements form? Web alkali metals and alkaline earth metals always form cations. Transition metals often form ions wihout complete octets that's why all the stable ions are all cations. It has fewer electrons than protons. And because of this behavior,.
All Chemical Reactions Are Driven To Gain More.
Web halogens always form anions, alkali metals and alkaline earth metals always form cations. Web cations are positively charged ions. Cl atoms gain 1 electron, therefore making them anions. Oxygen, nitrogen, sulfur), while most metals form cations (e.g.
By Catrin Brown And Mike Ford.
The solvation number , n , determined by a variety of experimental methods is 4 for li + and be 2+ and 6 for most elements in periods 3. Web alkali metals and alkaline earth metals always form cations. Transition metals often form ions wihout complete octets that's why all the stable ions are all cations. Closely packed cations and loosely held valence electrons rather than neutral atoms what can the valence electrons in a pure metal be modeled as?
Pearson Higher Level Chemistry Textbook, 2Nd Edition.
They are formed when a metal loses its electrons. Web cations are atoms that contain a positive charge, and they are formed when the atoms lose electrons which are negatively charged. Web having lost electrons, which are negatively charged, atoms of metals therefore gain a net positive charge i.e they become cations. Most other nonmetals typically form anions (e.g.
It Has Fewer Electrons Than Protons.
Web a metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m(h 2 o) n] z+. Writing chemical formulas when writing the formula of a compound, the cation is listed before the anion. Therefore, they possess a net positive charge. Web what do metals consist of?