Does Sodium And Fluorine Form An Ionic Compound
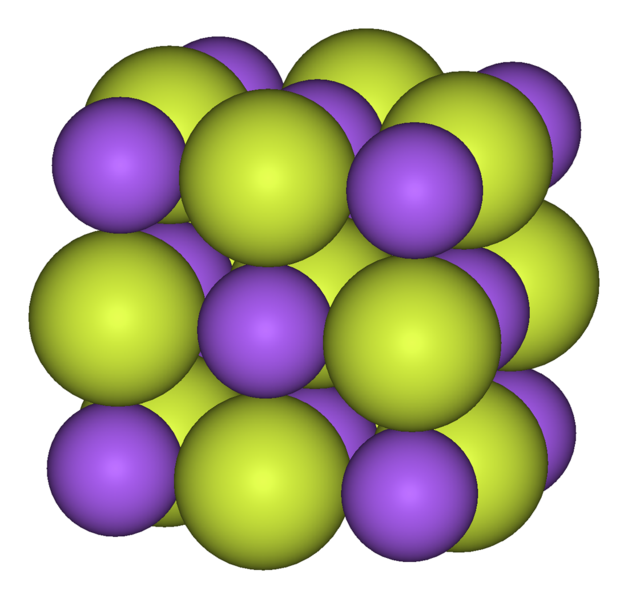
Does Sodium And Fluorine Form An Ionic Compound - The aluminum ion and the fluoride ion; An ionic bond is also known as electrovalent bond. Yellow is fluorine, purple is sodium. Can fluorine and sodium for a ionic compound? Since n a is a metal and f is a nonmetal, these two can combine together to form an ionic bond. Web fluorides are properly defined as binary compounds or salts of fluorine and another element. The sodium ion and the sulfur ion; Web here sodium transfers its one electron from itself to fluorine and thus an ionic bond is formed between them. We know that n a tends to have an. In this reaction, the sodium atom loses its single valence.
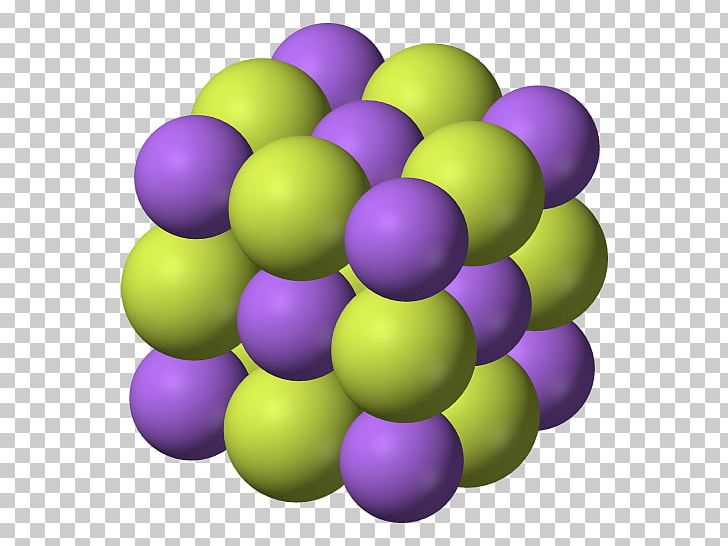
The sodium ion and the sulfur ion; The 3+ iron ion and. The alkali metals form monofluorides. Web fluorides are properly defined as binary compounds or salts of fluorine and another element. Examples of fluorides include sodium fluoride and calcium fluoride. The aluminum ion and the fluoride ion; Web a crystal of sodium chloride, shown here, is a collection of alternating sodium and chlorine ions. The formula for an ionic compound follows several conventions. Web since the fluoride ion is small (1.33 å) and the least polarizable anion (i.e., hard) it is stable in ionic lattices with metal cations in a high oxidation state (high charge), e.g., mnf 4 and. Thus n a f is.
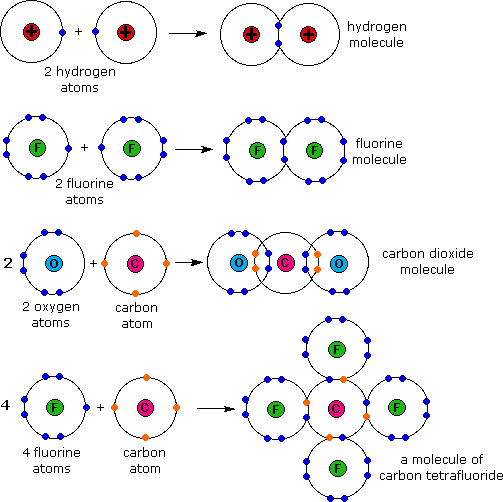
One example of an ionic bond is the formation of sodium fluoride, naf, from a sodium atom and a fluorine atom. The formula for an ionic compound follows several conventions. Web here sodium transfers its one electron from itself to fluorine and thus an ionic bond is formed between them. The sodium ion and the sulfur ion; Since n a is a metal and f is a nonmetal, these two can combine together to form an ionic bond. Examples of fluorides include sodium fluoride and calcium fluoride. They are isoelectronic, but fluorine is bigger because its nuclear charge is lower. Yellow is fluorine, purple is sodium. In this reaction, the sodium atom loses its single valence. An ionic bond is also known as electrovalent bond.
Ionic Bonding Stock Illustrations 65 Ionic Bonding Stock
An ionic bond is also known as electrovalent bond. Web since the fluoride ion is small (1.33 å) and the least polarizable anion (i.e., hard) it is stable in ionic lattices with metal cations in a high oxidation state (high charge), e.g., mnf 4 and. Web here sodium transfers its one electron from itself to fluorine and thus an ionic.
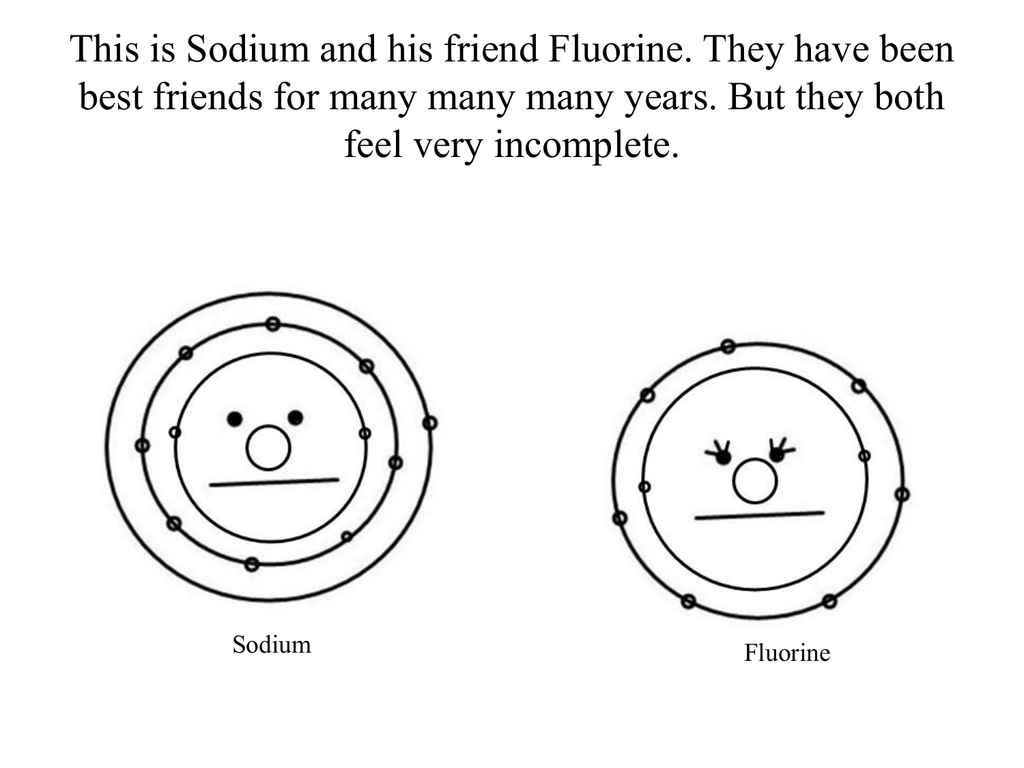
This is Sodium and his friend Fluorine. They have been
The alkali metals form monofluorides. Web one example of an ionic bond is the formation of sodium fluoride, naf, from a sodium atom and a fluorine atom. The formula for an ionic compound follows several conventions. Thus n a f is. One example of an ionic bond is the formation of sodium fluoride, naf, from a sodium atom and a.
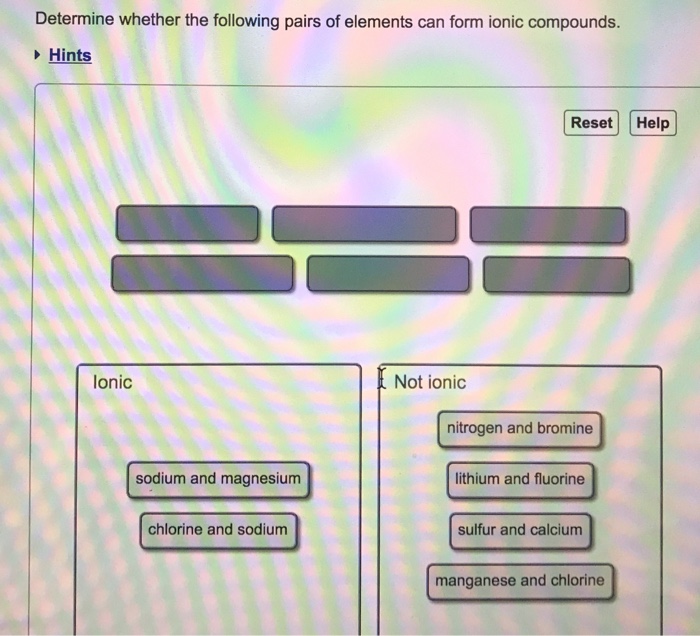
Solved Determine Whether The Following Pairs Of Elements
Examples of fluorides include sodium fluoride and calcium fluoride. The alkali metals form monofluorides. Since n a is a metal and f is a nonmetal, these two can combine together to form an ionic bond. Can fluorine and sodium for a ionic compound? Web here sodium transfers its one electron from itself to fluorine and thus an ionic bond is.
Solved 5. Sulfur and fluorine combine to form compounds that
The aluminum ion and the fluoride ion; In this reaction, the sodium atom loses its single valence. We know that n a tends to have an. The alkali metals form monofluorides. Web fluorides are properly defined as binary compounds or salts of fluorine and another element.
The Periodic Table and Bonding Mrs. Sanborn's Site
The formula for an ionic compound follows several conventions. Web does fluorine and sodium form an ionic compound? In this reaction, the sodium atom loses its single valence. Can fluorine and sodium for a ionic compound? Yellow is fluorine, purple is sodium.
Sodium Fluoride Ionic Bonding Crystal Structure PNG, Clipart, Chemical
Web sodium flouride explanation: The aluminum ion and the fluoride ion; Web fluorides are properly defined as binary compounds or salts of fluorine and another element. One example of an ionic bond is the formation of sodium fluoride, naf, from a sodium atom and a fluorine atom. Yellow is fluorine, purple is sodium.
Fluorine (F) Properties & Uses StudiousGuy
Web here sodium transfers its one electron from itself to fluorine and thus an ionic bond is formed between them. They are isoelectronic, but fluorine is bigger because its nuclear charge is lower. One example of an ionic bond is the formation of sodium fluoride, naf, from a sodium atom and a fluorine atom. Web write the chemical formula for.
Gradegorilla GCSE Chemistry
Web one example of an ionic bond is the formation of sodium fluoride, naf, from a sodium atom and a fluorine atom. They are isoelectronic, but fluorine is bigger because its nuclear charge is lower. Web since the fluoride ion is small (1.33 å) and the least polarizable anion (i.e., hard) it is stable in ionic lattices with metal cations.
halogen Facts, Definition, Properties, & Uses Britannica
The 3+ iron ion and. Web since the fluoride ion is small (1.33 å) and the least polarizable anion (i.e., hard) it is stable in ionic lattices with metal cations in a high oxidation state (high charge), e.g., mnf 4 and. Web solid metallic elemental sodium (na) will combine with pure gaseous elemental fluorine (f2) to produce a simple binary.
Fluoride Powders
Web a crystal of sodium chloride, shown here, is a collection of alternating sodium and chlorine ions. Thus n a f is. Since n a is a metal and f is a nonmetal, these two can combine together to form an ionic bond. Web one example of an ionic bond is the formation of sodium fluoride, naf, from a sodium.
Can Fluorine And Sodium For A Ionic Compound?
Web since the fluoride ion is small (1.33 å) and the least polarizable anion (i.e., hard) it is stable in ionic lattices with metal cations in a high oxidation state (high charge), e.g., mnf 4 and. Web solid metallic elemental sodium (na) will combine with pure gaseous elemental fluorine (f2) to produce a simple binary ionic compound (sodium fluoride,. Web sodium flouride explanation: Thus n a f is.
In This Reaction, The Sodium Atom Loses Its Single Valence.
Web does fluorine and sodium form an ionic compound? The sodium ion and the sulfur ion; Web here sodium transfers its one electron from itself to fluorine and thus an ionic bond is formed between them. Web one example of an ionic bond is the formation of sodium fluoride, naf, from a sodium atom and a fluorine atom.
Examples Of Fluorides Include Sodium Fluoride And Calcium Fluoride.
Web a crystal of sodium chloride, shown here, is a collection of alternating sodium and chlorine ions. The 3+ iron ion and. The aluminum ion and the fluoride ion; Since n a is a metal and f is a nonmetal, these two can combine together to form an ionic bond.
The Formula For An Ionic Compound Follows Several Conventions.
We know that n a tends to have an. Yellow is fluorine, purple is sodium. They are isoelectronic, but fluorine is bigger because its nuclear charge is lower. One example of an ionic bond is the formation of sodium fluoride, naf, from a sodium atom and a fluorine atom.