How Does A Positive Ion Form
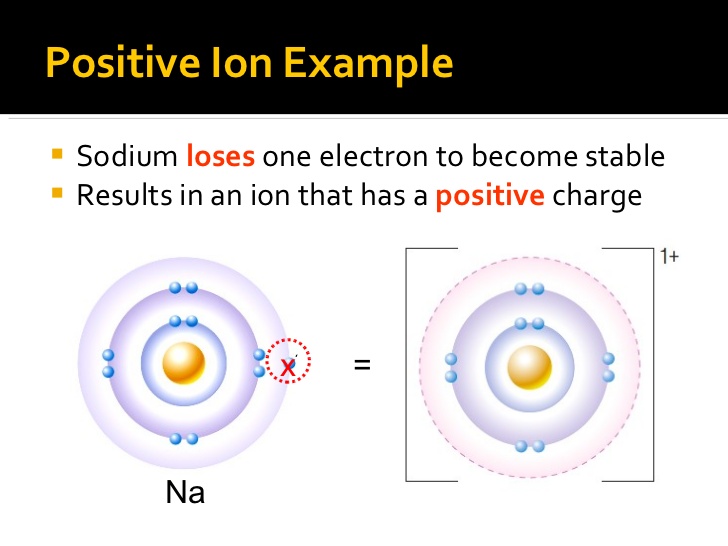
How Does A Positive Ion Form - All electrons in the outer energy level are lost. Web a positive ion is formed by the loss of negatively charged electrons. Although the number of protons does not change in the ion, there is an excess number of protons over electrons which produces the positive charge. It has one electron in its valence shell, which makes it unstable. Web a positive ion is formed when an atom of a metal loses one or more electrons. Web formation of ions in ordinary chemical reactions, the nucleus of each atom (and thus the element's identity) remains unchanged. The electrostatic attraction between the positives and negatives brings the particles together and creates an ionic compound, such as sodium chloride. An ion is an atom or molecule with a net electrical charge. What is an example of a positive ion? They form through ionic bonding.
They form through ionic bonding. Elements gain or lose electrons to form ions and to gain full outer shells. Do not equal the number of protons in the atom or molecule. Web forming positive ions metal atoms lose electrons from their outer shell when they form ions: Electrostatics explains why this happens: This gives the metal ion a filled valence shell. Web forming positive ions (cations) atoms lose electrons from their outer shell when they form positive ions, called cations. Many common materials contain these ions. These oppositely charged ions attract each other to form ionic networks (or lattices ). Web what is the proper definition of ions?
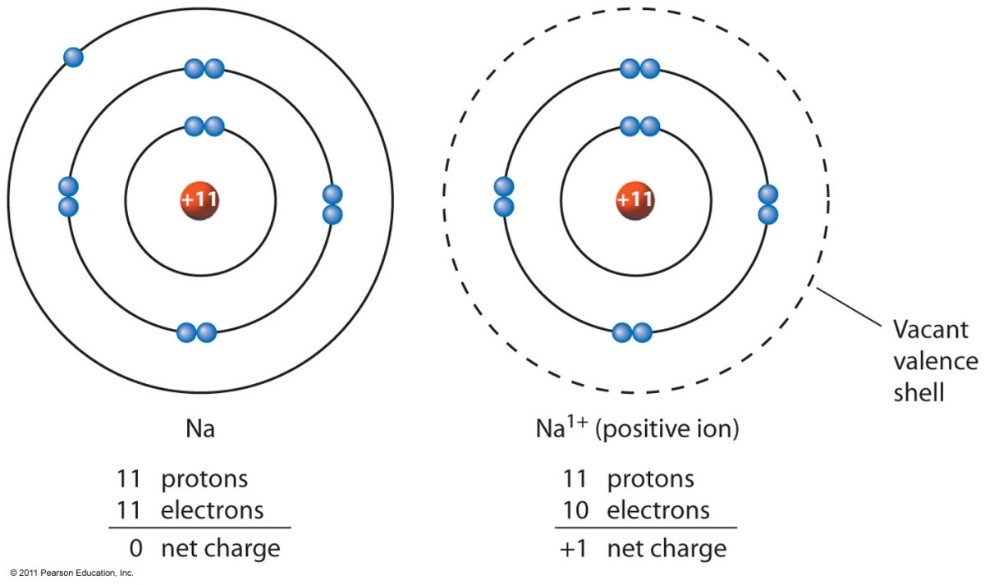
Elements gain or lose electrons to form ions and to gain full outer shells. These ions are positive because they contain more protons. Web cations are the positive ions formed by the loss of one or more electrons. The degree of ionization of the plasma depends strongly on the electron density and energy distribution in the gas. Do not equal the number of protons in the atom or molecule. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. Ions migrate under the influence of an electrical field and are the conductors of electric current in electrolytic cells. Web if there are more electrons, the atom will form a negative ion, but if the atom has more protons, the atom will form a positive ion. Web jun 3, 2018 see below. Electrostatics explains why this happens:
What happens if an atom is not neutral? Socratic
Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. The most commonly formed cations of the representative elements are those that involve the loss of all of the valence electrons. Web what is the proper definition of ions? The net charge of an ion is not zero because its total number.
PPT How do atoms form ions? PowerPoint Presentation ID7021047
Web formation of ions in ordinary chemical reactions, the nucleus of each atom (and thus the element's identity) remains unchanged. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The degree of ionization of the plasma.
NH4+ Lewis Structure (Ammonium Ion) Math, Lewis, Positivity
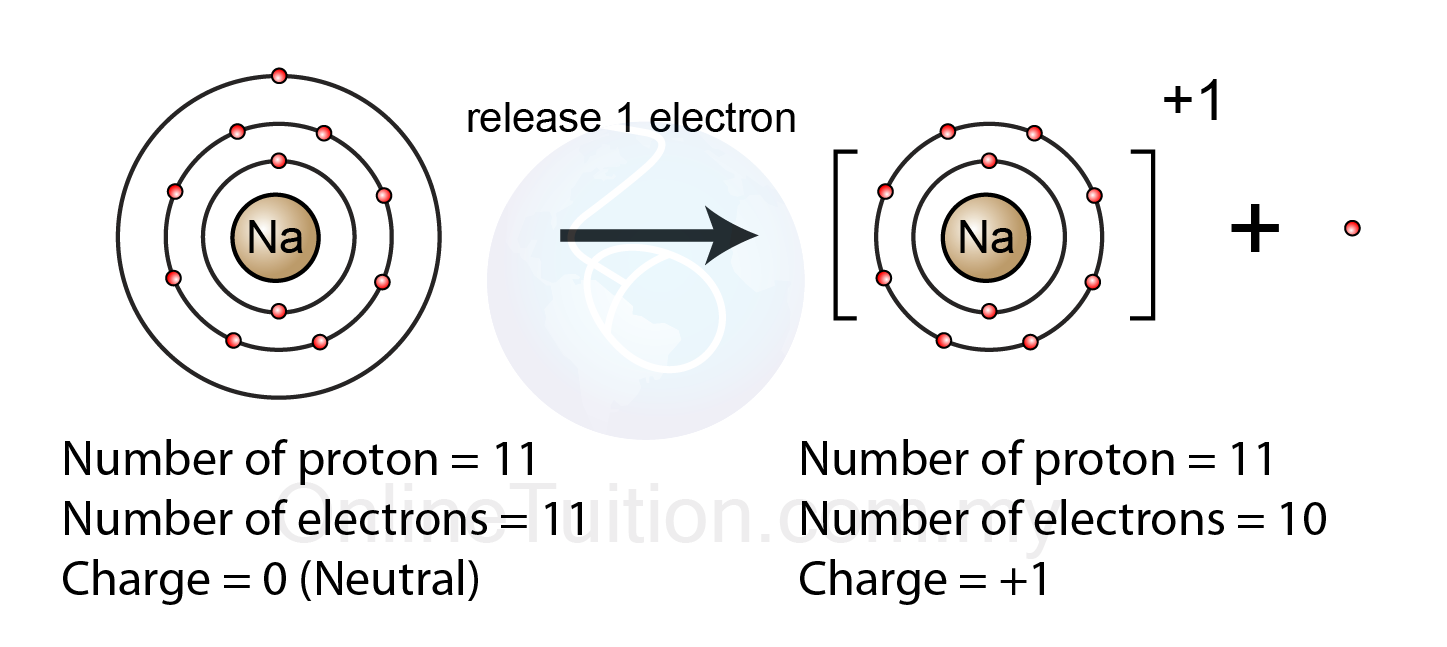
The electrostatic attraction between the positives and negatives brings the particles together and creates an ionic compound, such as sodium chloride. Web a positive ion forms when an atom loses one or more valence electron. Examples of positive ions positive ions are typically metals or act like metals. The net charge of an ion is not zero because its total.
Ions Types, Summary, Classification & Facts
Web a positive ion is formed when an atom of a metal loses one or more electrons. An ion is a charged atom or molecule. Positively charged ions are called cations; Web forming positive ions metal atoms lose electrons from their outer shell when they form ions: Examples of positive ions positive ions are typically metals or act like metals.
5.2.1 Formation of Ion Revision.my
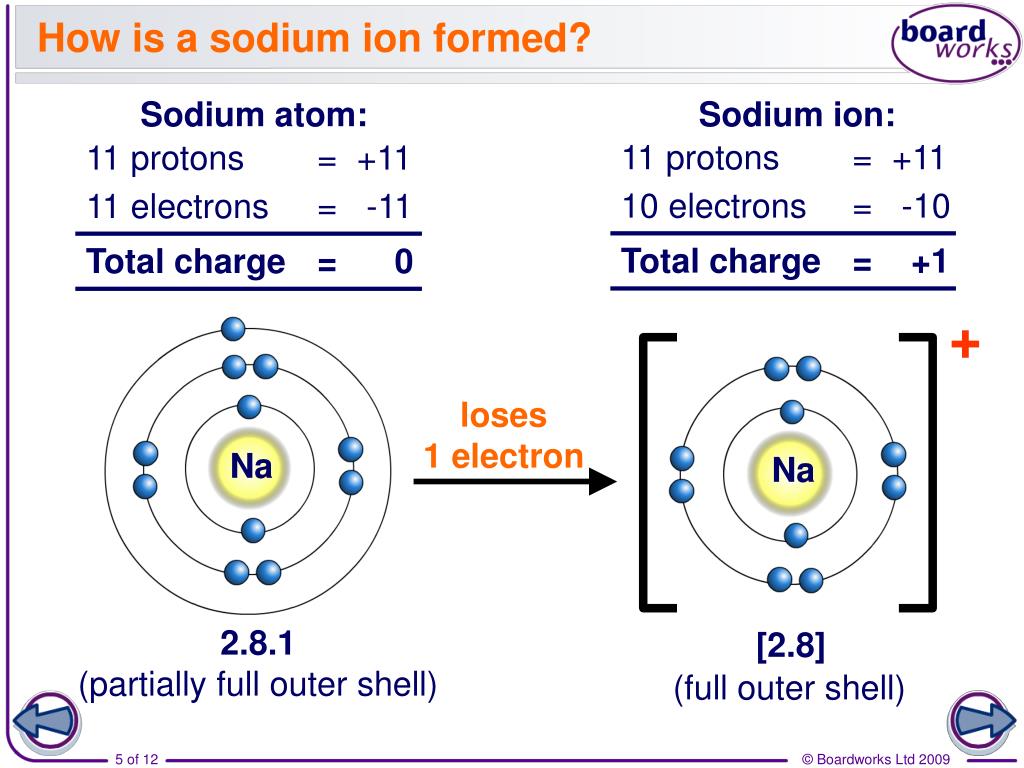
Although the number of protons does not change in the ion, there is an excess number of protons over electrons which produces the positive charge. Do not equal the number of protons in the atom or molecule. Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Electrostatics explains why this happens:.
3.2 Ions The Basics of General, Organic, and Biological Chemistry
Opposite charges attract and like charges repel. Web a positive ion is formed when an atom of a metal loses one or more electrons. All electrons in the outer energy level are lost. The transfer and sharing of electrons among atoms govern the chemistry of the. The degree of ionization of the plasma depends strongly on the electron density and.
Ions
Web if there are more electrons, the atom will form a negative ion, but if the atom has more protons, the atom will form a positive ion. Examples of positive ions positive ions are typically metals or act like metals. Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Web a.
Ions — Definition & Overview Expii
An atom can acquire a positive charge or a negative charge depending on whether the number of electrons Web a positive ion is formed when an atom of a metal loses one or more electrons. Opposite charges attract and like charges repel. Electrons in the outer level. Web a positive ion forms when an atom loses one or more valence.
Molecular and Ionic Compounds Chemistry I
When oppositely charged ions are brought Positively charged ions are called cations; Consider the alkali metal sodium ( na). For example, let's look at lithium and fluorine: It has one valence electron in the n = 3 energy level.
What Does Ion Mean? Slanguide
The most commonly formed cations of the representative elements are those that involve the loss of all of the valence electrons. Web formation of ions in ordinary chemical reactions, the nucleus of each atom (and thus the element's identity) remains unchanged. Web a positive ion is formed when an atom of a metal loses one or more electrons. Do not.
An Ion Is An Atom Or Molecule With A Net Electrical Charge.
Web at r0, the ions are more stable (have a lower potential energy) than they are at an infinite internuclear distance. They form through ionic bonding. Web a positive ion is formed by the loss of negatively charged electrons. Electrons in the outer level.
Lithium Has 3 Electrons, So.
The ions are positive, because they have more protons than electrons the ions formed have. All electrons in the outer energy level are lost. The degree of ionization of the plasma depends strongly on the electron density and energy distribution in the gas. Web positive ions are formed by atoms or molecules suffering an inelastic collision with an energetic electron in which an electron is lost from the atom or molecule (electron impact ionization).
The Net Charge Of An Ion Is Not Zero Because Its Total Number Of Electrons Is Unequal To Its Total Number Of Protons.
Opposite charges attract and like charges repel. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. This gives the metal ion a filled valence shell.
The Electrostatic Attraction Between The Positives And Negatives Brings The Particles Together And Creates An Ionic Compound, Such As Sodium Chloride.
For example, let's look at lithium and fluorine: The transfer and sharing of electrons among atoms govern the chemistry of the. Web a positive ion is formed when an atom of a metal loses one or more electrons. Web jun 3, 2018 see below.