How Many Hydrogen Bonds Can Water Form
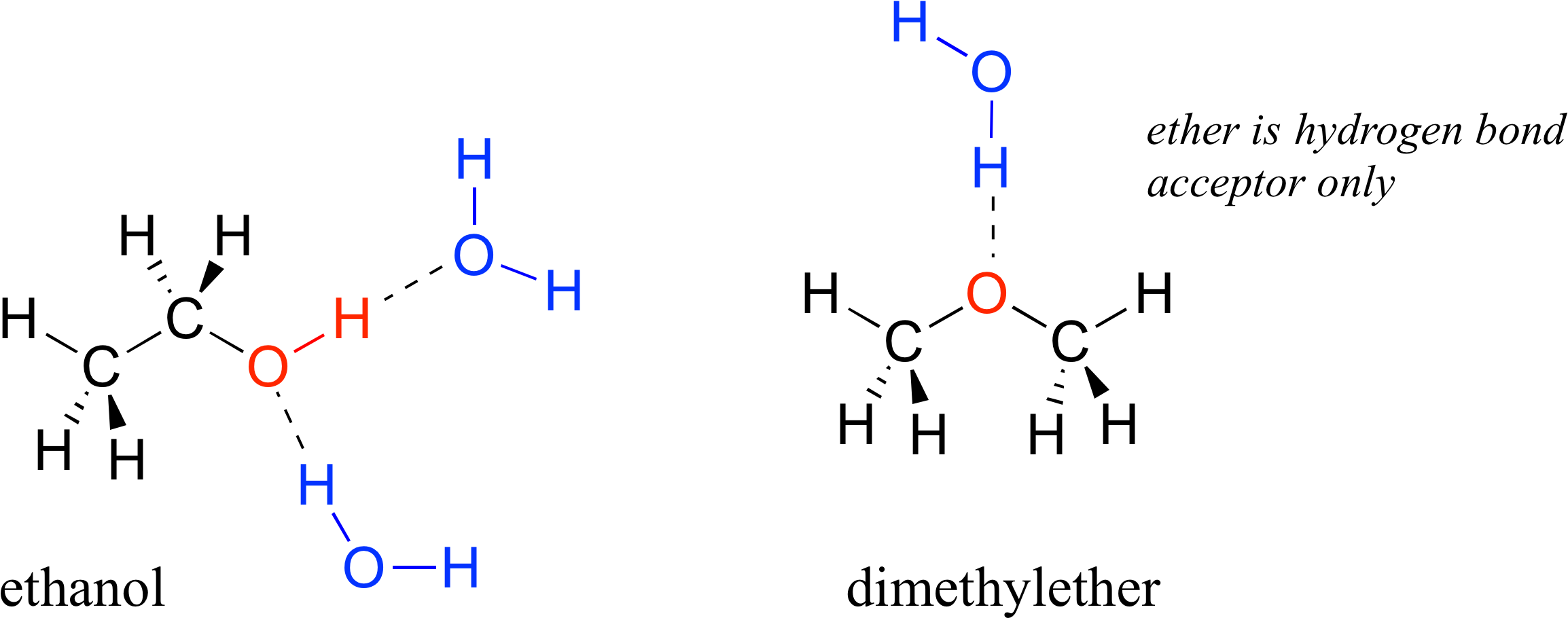
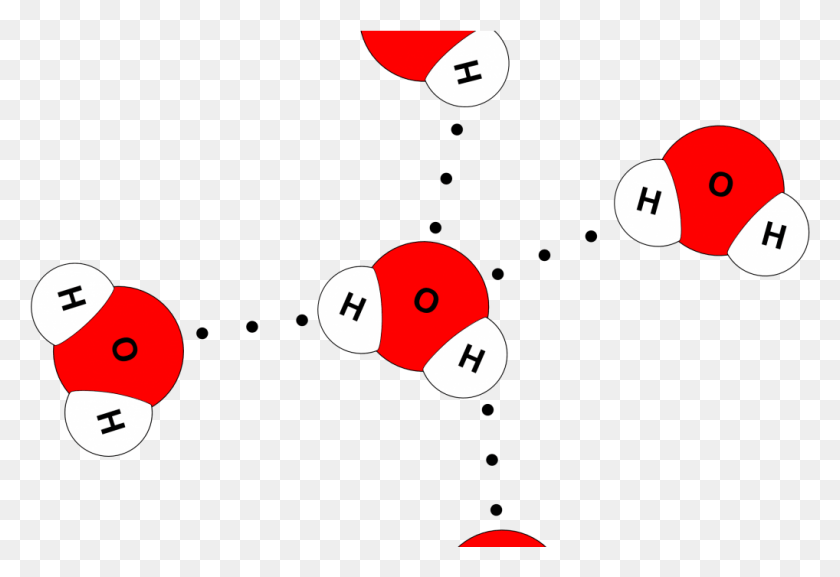
How Many Hydrogen Bonds Can Water Form - Web water is can ideally model of hydrogen stick. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Web water can form four hydrogen bonds. Web in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. 1 answer evan holbrook jun 21, 2018 due to the large difference in electronegativity between oxygen. Web the 4 possible hydrogen bonds formed with a water molecule in ice. The two lone pairs of oxygen atoms and the two hydrogen atoms of water are involved in intermolecular hydrogen bonding. The number of hydrogen bonds formed/molecule in liquid water is less than four, and decreases as the. There are exactly the right numbers of + hydrogens. Notice that everyone water molecule can potentially form four hydrogen debenture the surrounds aquarium molecules:
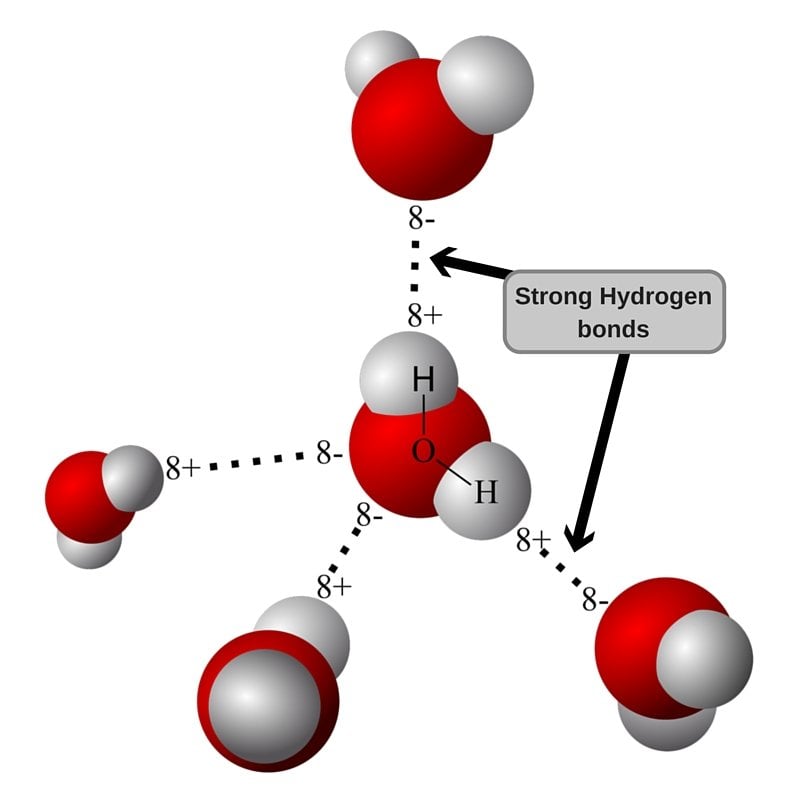
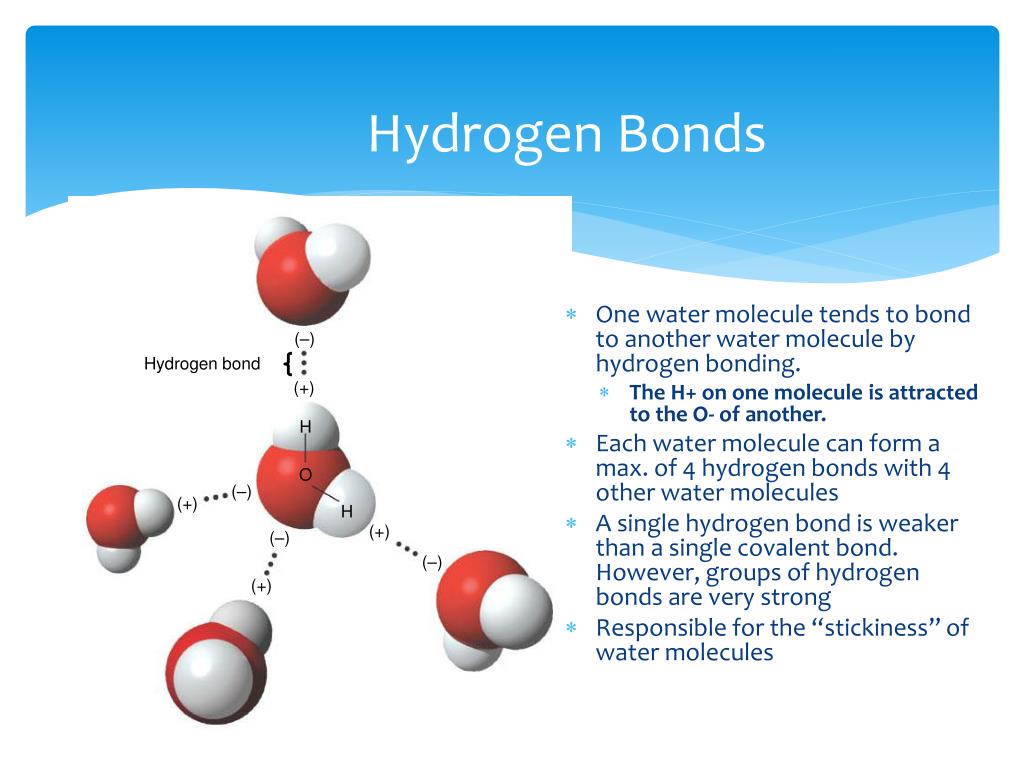
There are exactly the right numbers of + hydrogens. Web in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Both an oxygen atom and 2 hydrogen atoms in one molecule. Water (h 2 o) is a simple triatomic bent molecule with c 2v molecular symmetry and bond angle of 104.5°. Web up to 4 hydrogen bonds can form between a single water molecule and other water molecules. Both of these atoms can form a hydrogen bond with oxygen atoms of different water molecules. Web why does water form hydrogen bonds? Web water is unique because its oxygen atom has two lone pairs and two hydrogen atoms, meaning that the total number of bonds of a water molecule is up to four. Web lewis structure of h 2 o indicating bond angle and bond length. The two lone pairs of oxygen atoms and the two hydrogen atoms of water are involved in intermolecular hydrogen bonding.
1 answer evan holbrook jun 21, 2018 due to the large difference in electronegativity between oxygen. Web up to 4 hydrogen bonds can form between a single water molecule and other water molecules. Web in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. The number of hydrogen bonds formed/molecule in liquid water is less than four, and decreases as the. Notice that everyone water molecule can potentially form four hydrogen debenture the surrounds aquarium molecules: Web water is can ideally model of hydrogen stick. There are exactly the right numbers of + hydrogens. Both an oxygen atom and 2 hydrogen atoms in one molecule. In h 2 o, only two of the six. Every water molecule can be.
Bonds That Hold Water Molecules Together / Intermolecular Forces
Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Web lewis structure of h 2 o indicating bond angle and bond length. The number of hydrogen bonds formed/molecule in liquid water is less than four, and decreases as the. Both an oxygen atom and 2 hydrogen atoms in one molecule. Both of these.
Download How Many Hydrogen Bonds Can A Water Molecule Form Hydrogen
Water (h 2 o) is a simple triatomic bent molecule with c 2v molecular symmetry and bond angle of 104.5°. Web the 4 possible hydrogen bonds formed with a water molecule in ice. Web water can form four hydrogen bonds. Web in the liquid phase, the average number of hydrogen bonds that a water molecule participates in varies greatly with.
Why Do Fingers/Hands Stick To Ice? » Science ABC
Web water is unique because its oxygen atom has two lone pairs and two hydrogen atoms, meaning that the total number of bonds of a water molecule is up to four. 1 answer evan holbrook jun 21, 2018 due to the large difference in electronegativity between oxygen. Notice that everyone water molecule can potentially form four hydrogen debenture the surrounds.
Difference Between Intermolecular and Intramolecular Hydrogen Bonding
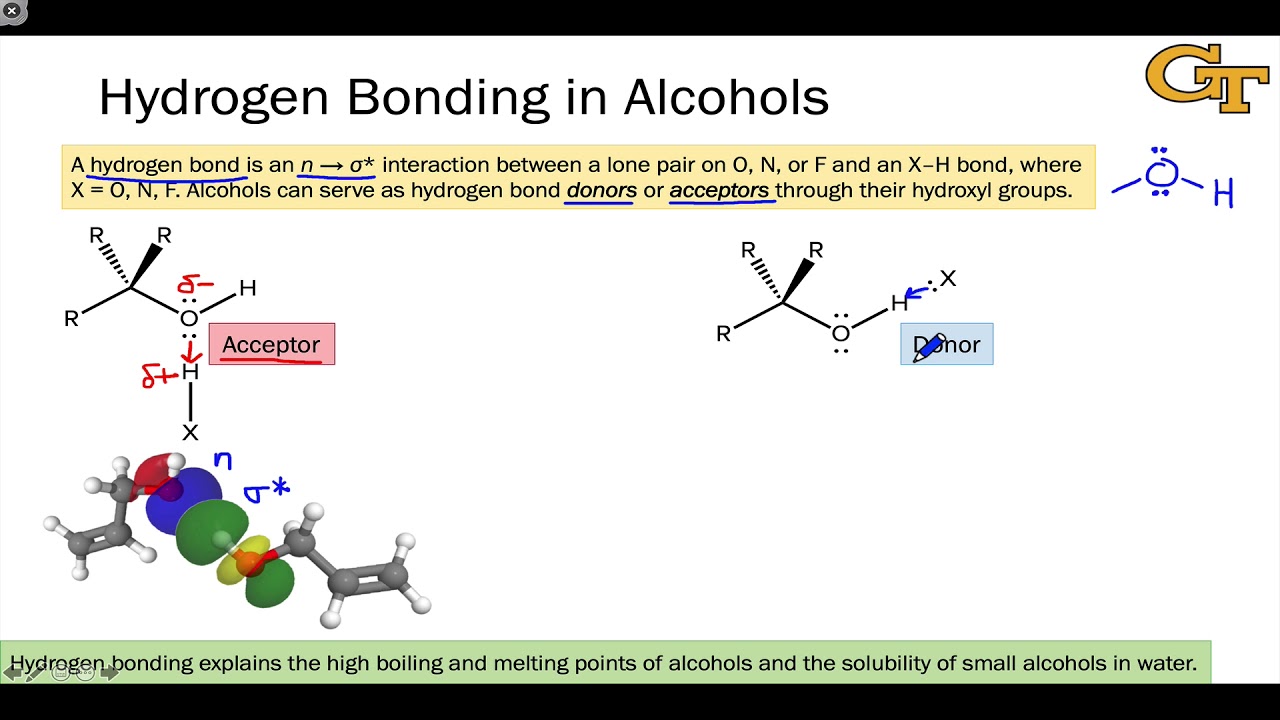
Web in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. There are exactly the right numbers of + hydrogens. Both an oxygen atom and 2 hydrogen atoms in one molecule. Web a molecule of water has two hydrogen atoms. Web water can form four hydrogen bonds.
Hexanol Model Molecule Carbon 3d Ball Stick Volatile Organic Compounds
Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Web a molecule of water has two hydrogen atoms. There are exactly the right numbers of + hydrogens. Web lewis structure of h 2 o indicating bond angle and bond length. Web why does water form hydrogen bonds?
Water
Web water is unique because its oxygen atom has two lone pairs and two hydrogen atoms, meaning that the total number of bonds of a water molecule is up to four. Both of these atoms can form a hydrogen bond with oxygen atoms of different water molecules. Web in the liquid phase, the average number of hydrogen bonds that a.
How Many Hydrogen Bonds Can Ethanol Form Printable Form, Templates
Web in the liquid phase, the average number of hydrogen bonds that a water molecule participates in varies greatly with temperature, the average being 3.69 at 0ºc,. Web water can form four hydrogen bonds. Web in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Both of.
How many hydrogen bonds are attached to each water molecule in a solid
Web in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. The two lone pairs of oxygen atoms and the two hydrogen atoms of water are involved in intermolecular hydrogen bonding. Web water is unique because its oxygen atom has two lone pairs and two hydrogen atoms,.
PPT Properties of Water PowerPoint Presentation, free download ID
The two lone pairs of oxygen atoms and the two hydrogen atoms of water are involved in intermolecular hydrogen bonding. Web in the liquid phase, the average number of hydrogen bonds that a water molecule participates in varies greatly with temperature, the average being 3.69 at 0ºc,. Web notice that each water molecule can potentially form four hydrogen bonds with.
How many hydrogen bonds a water molecule can form Hydrogen Bonding in
Web lewis structure of h 2 o indicating bond angle and bond length. Water (h 2 o) is a simple triatomic bent molecule with c 2v molecular symmetry and bond angle of 104.5°. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. Web in water, each hydrogen nucleus is covalently bound to the.
Web In Water, Each Hydrogen Nucleus Is Covalently Bound To The Central Oxygen Atom By A Pair Of Electrons That Are Shared Between Them.
Web water is unique because its oxygen atom has two lone pairs and two hydrogen atoms, meaning that the total number of bonds of a water molecule is up to four. Web a molecule of water has two hydrogen atoms. Web answer (1 of 15): Web water can form four hydrogen bonds.
Notice That Everyone Water Molecule Can Potentially Form Four Hydrogen Debenture The Surrounds Aquarium Molecules:
Web up to 4 hydrogen bonds can form between a single water molecule and other water molecules. Web the 4 possible hydrogen bonds formed with a water molecule in ice. The two lone pairs of oxygen atoms and the two hydrogen atoms of water are involved in intermolecular hydrogen bonding. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules.
Web In The Liquid Phase, The Average Number Of Hydrogen Bonds That A Water Molecule Participates In Varies Greatly With Temperature, The Average Being 3.69 At 0ºc,.
Web water is can ideally model of hydrogen stick. Every water molecule can be. There are exactly the right numbers of + hydrogens. Web why does water form hydrogen bonds?
1 Answer Evan Holbrook Jun 21, 2018 Due To The Large Difference In Electronegativity Between Oxygen.
Water (h 2 o) is a simple triatomic bent molecule with c 2v molecular symmetry and bond angle of 104.5°. In h 2 o, only two of the six. Both of these atoms can form a hydrogen bond with oxygen atoms of different water molecules. It makes two with the.