Neon Electron Configuration Long Form
Neon Electron Configuration Long Form - Web using aufbau principle. Web electronic configuration of neon = 2, 8.neon is a chemical element with symbol ne and atomic number 10. 1s 2 2s 2 2p 6. ← electronic configurations of elements. The electron configurations and orbital. Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. The n=1 shell can only hold 2 electrons, so the remaining 8. The remaining electron must occupy the orbital. Possible oxidation states are 0. Web neon electron configuration number.
← electronic configurations of elements. In order to write the ne electron configuration we first need to know the number of elect show. Web electronic configuration of neon (ne): Web the complete electron configuration of neon is 1s 2 2s 2 2p 6.neon has 8 valence electrons around the nucleus and the atomic number is 10. Located in the ii period. Web the first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium. Possible oxidation states are 0. Web neon is the tenth element with a total of 10 electrons. In writing the electron configuration for neon the first two electrons will go in the 1s orbital. The electron configurations and orbital.
Web neon has atomic number 10, so a neon atom has 10 protons in its nucleus and therefore 10 electrons. Web electron configuration of neon is [he] 2s2 2p6. ← electronic configurations of elements. The atomic number of neon represents the total number of electrons of neon. Possible oxidation states are 0. In order to write the ne electron configuration we first need to know the number of elect show. Possible oxidation states are 0. Web neon is the tenth element with a total of 10 electrons. Neon is a very inert element, however, it has been reported to form a compound with fluorine. It is in group 18(noble gases) of the periodic table.
Electron configurations
Web using aufbau principle. For each atom the subshells are given first in concise form, then with all. The atomic number of neon represents the total number of electrons of neon. In order to write the ne electron configuration we first need to know the number of elect show. Web the complete electron configuration of neon is 1s 2 2s.
Neon Element (Ne 10) of Periodic Table Periodic Table FlashCard
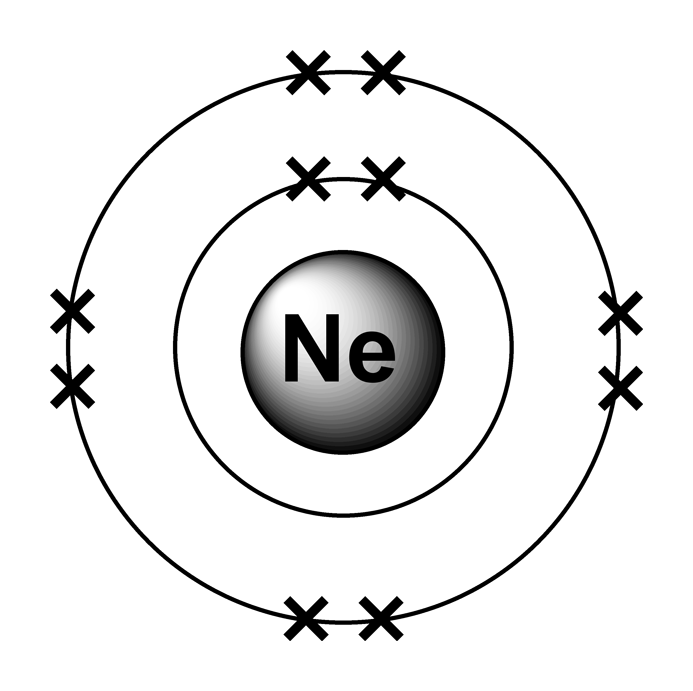
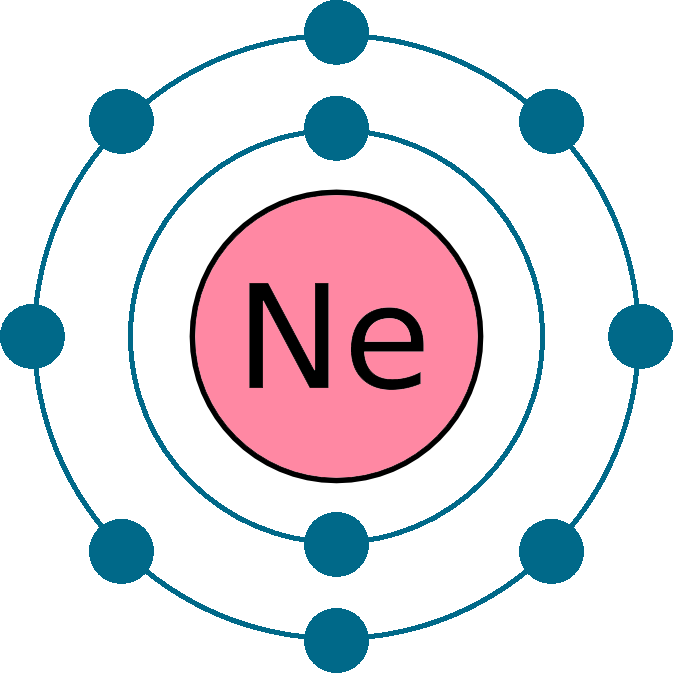
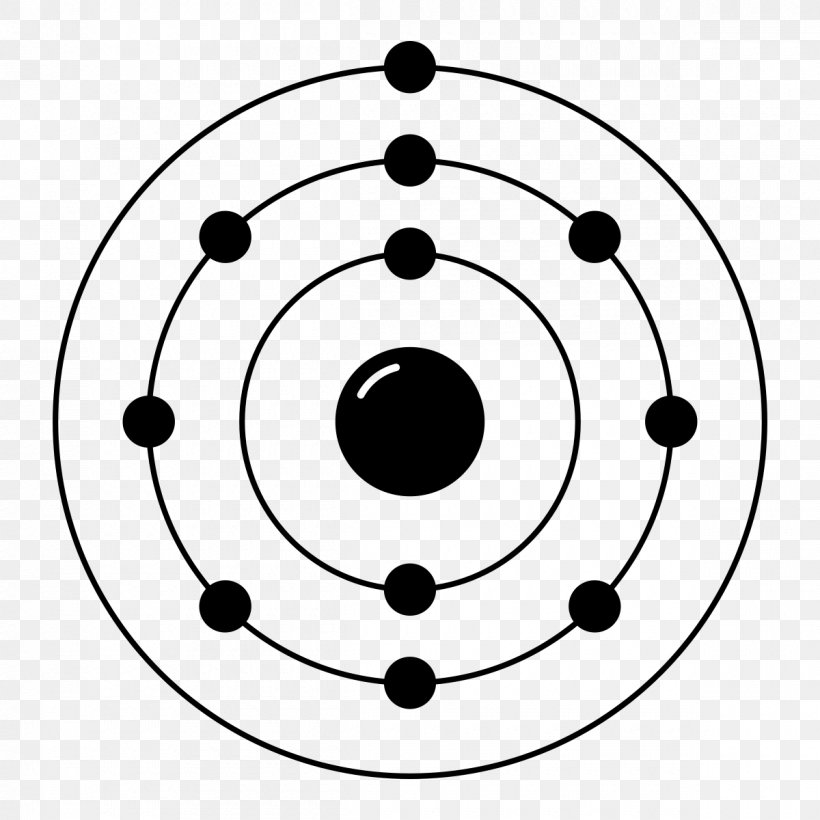
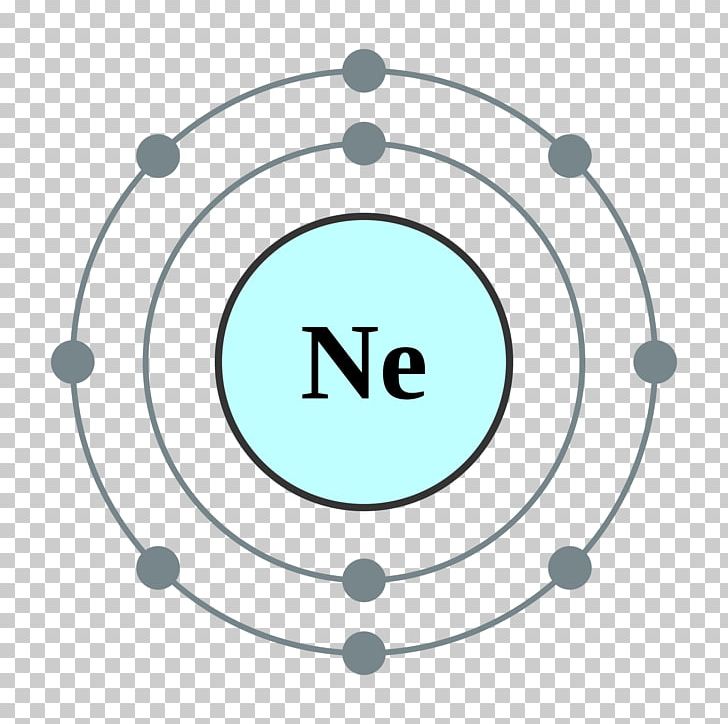
Ne (neon) is an element with position number 10 in the periodic table. Web neon electron configuration number. Web electronic configuration of neon (ne): Web electronic configuration of neon = 2, 8.neon is a chemical element with symbol ne and atomic number 10. Density of neon density of neon is 0.0009g/cm3.
Neon Electron Configuration Bohr Model Electron Shell Noble Gas, PNG
The electronic configuration of neon is ne: Web electron configuration of neon is [he] 2s2 2p6. Web electron configuration of neon is [he] 2s2 2p6. In order to write the ne electron configuration we first need to know the number of elect show. Neon is a very inert element, however, it has been reported to form a compound with fluorine.
Neon Electron Configuration Noble Gas Valence Electron Lewis Structure
Neon is a very inert element, however, it has been reported to form a compound with fluorine. Since the atomic number of neon is 10, the total electrons. Possible oxidation states are 0. ← electronic configurations of elements. Web using aufbau principle.
Electron Configuration Noble Gas Neon Electron Shell, PNG, 600x600px
Typical densities of various substances are at. Web the first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum numbers as the two electrons in helium. For each atom the subshells are given first in concise form, then with all. Ne (neon) is an element with position number 10 in the periodic.
Neon(Ne) electron configuration and orbital diagram
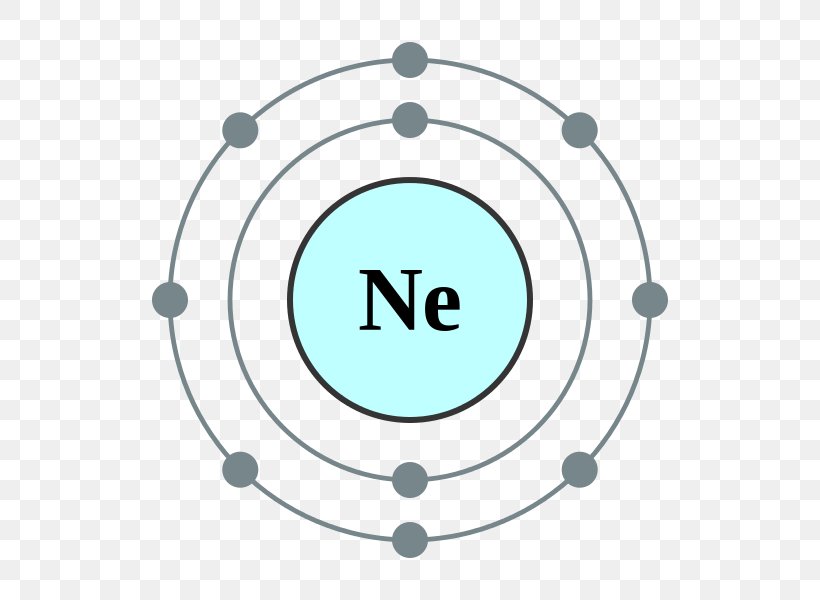
Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. Neon is a very inert element, however, it has been reported to form a compound with fluorine. Web electronic configuration of neon (ne): Located in the ii.
47 A ELECTRON CONFIGURATION OF NEON * ElectronConfiguration
It is in group 18(noble gases) of the periodic table. Since the atomic number of neon is 10, the total electrons. Web electronic configuration of neon = 2, 8.neon is a chemical element with symbol ne and atomic number 10. Web the first two electrons in lithium fill the 1 s orbital and have the same sets of four quantum.
Electron Configuration for Neon (Ne) Full Explanation
The configuration number of the electron is nothing but the same equation that represents the configuration for the. In order to write the ne electron configuration we first need to know the number of elect show. The n=1 shell can only hold 2 electrons, so the remaining 8. Web the complete electron configuration of neon is 1s 2 2s 2.
[5 Steps] Electron Configuration for or of Neon in Just 5 Steps
Density of neon density of neon is 0.0009g/cm3. The electronic configuration of neon is ne: So, the electron configuration of neon is 1 s 2 2 s 2 2 p 6 now, the neon. The remaining electron must occupy the orbital. Possible oxidation states are 0.
The Compound Neon Electron Configuration Scholars Ark
The electronic configuration of neon is ne: Web the complete electron configuration of neon is 1s 2 2s 2 2p 6.neon has 8 valence electrons around the nucleus and the atomic number is 10. It is still questionable if true compounds of neon exist, but. 1s 2 2s 2 2p 6. The atomic number of neon represents the total number.
Web Neon Electron Configuration Number.
The remaining electron must occupy the orbital. Web all of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. Web electronic configuration of neon (ne): 1s 2 2s 2 2p 6.
Web Electronic Configuration Of Neon = 2, 8.Neon Is A Chemical Element With Symbol Ne And Atomic Number 10.
Located in the ii period. It is still questionable if true compounds of neon exist, but. Neon is a very inert element, however, it has been reported to form a compound with fluorine. The n=1 shell can only hold 2 electrons, so the remaining 8.
The Electron Configurations And Orbital.
Since 1s can only hold two. Possible oxidation states are 0. Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. For each atom the subshells are given first in concise form, then with all.
It Is In Group 18(Noble Gases) Of The Periodic Table.
Web neon has atomic number 10, so a neon atom has 10 protons in its nucleus and therefore 10 electrons. The atomic number of neon represents the total number of electrons of neon. The configuration number of the electron is nothing but the same equation that represents the configuration for the. Since the atomic number of neon is 10, the total electrons.



![[5 Steps] Electron Configuration for or of Neon in Just 5 Steps](https://2.bp.blogspot.com/-jMs9FCVMeSw/XD4FtZiSVlI/AAAAAAAAYZg/rJnlLKM6S6EHv8FmJZr4pj2PmzTZjk0hQCLcBGAs/s1600/20190115_220744.jpg)
