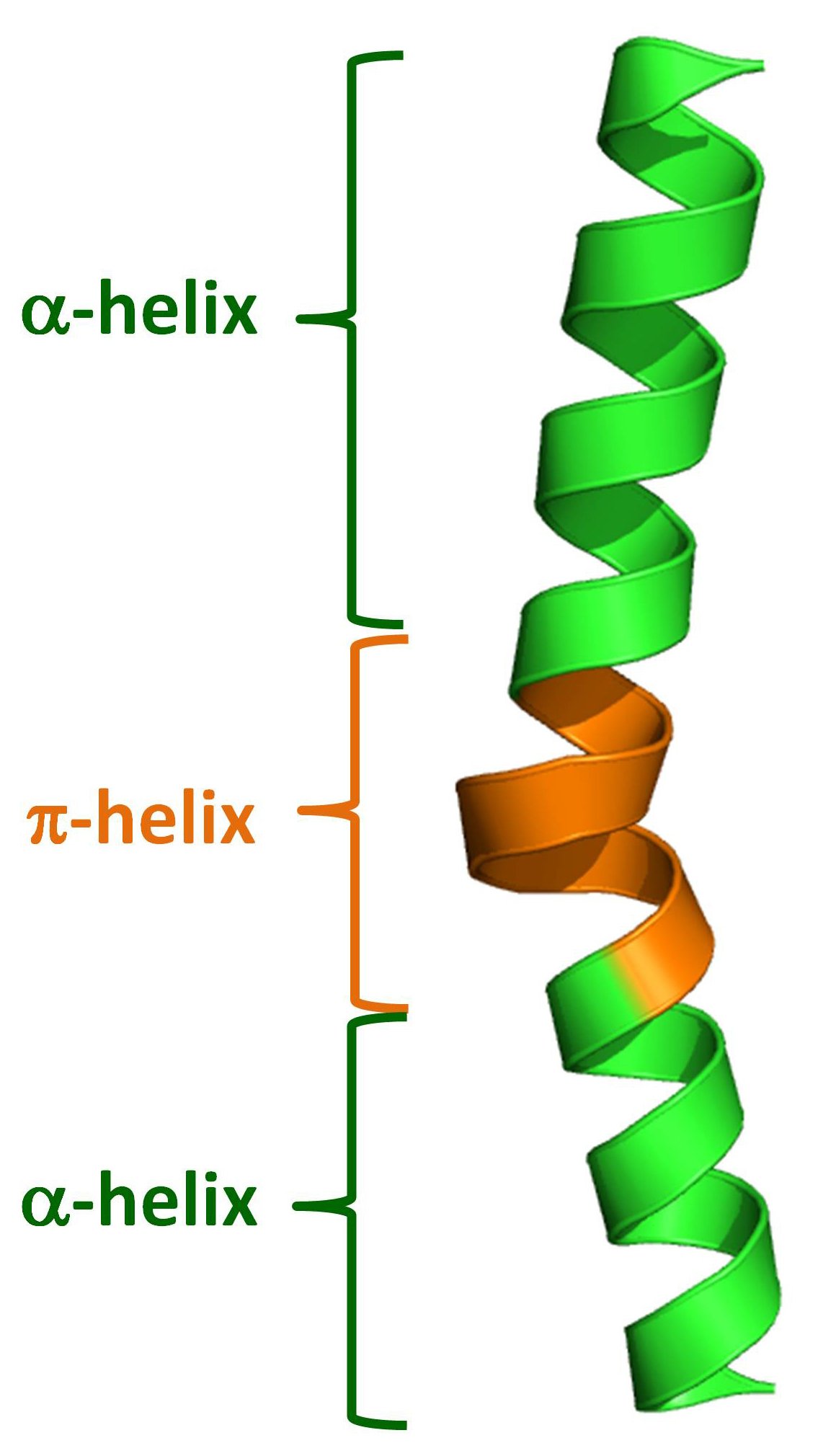
The Alpha-Helix And Beta-Pleated Sheet Are Characteristic Of
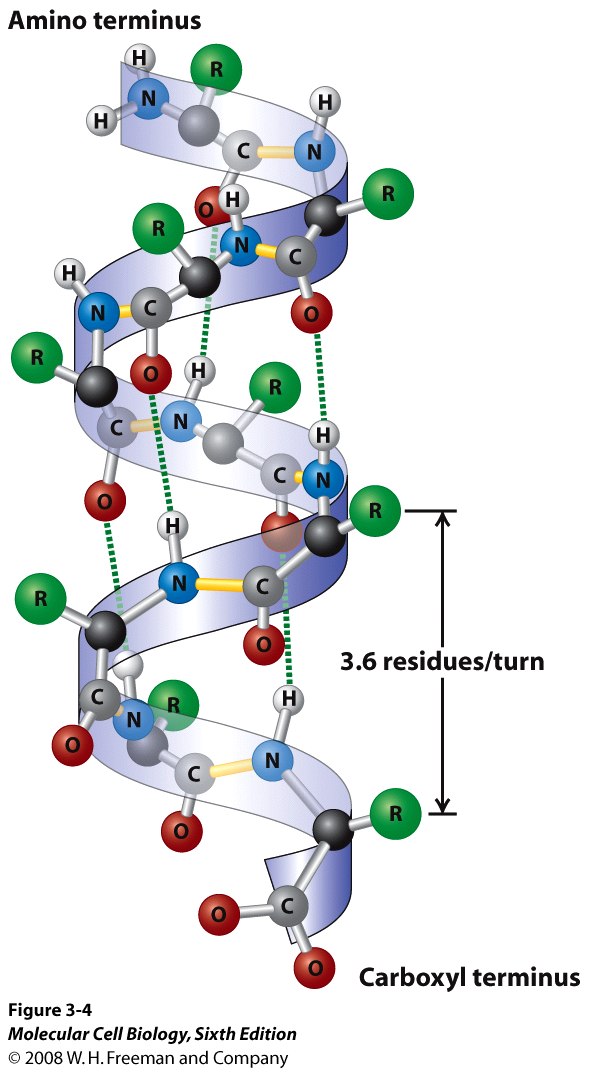
The Alpha-Helix And Beta-Pleated Sheet Are Characteristic Of - Web the most common types of secondary structures are the α helix and the β pleated sheet. A helix with 2 residues/turn. The arrangement of each successive peptide plane is pleated due to the tetrahedral nature of the alpha. Both structures are held in shape by hydrogen bonds, which form between the carbonyl o of one amino acid and the. The other portions of the polymer backbone that are regular but not repetitive are called. They both are shaped by hydrogen bonding between the carbonyl o of one amino acid and the amino h of another.
They both are shaped by hydrogen bonding between the carbonyl o of one amino acid and the amino h of another. A helix with 2 residues/turn. The arrangement of each successive peptide plane is pleated due to the tetrahedral nature of the alpha. Both structures are held in shape by hydrogen bonds, which form between the carbonyl o of one amino acid and the. Web the most common types of secondary structures are the α helix and the β pleated sheet. The other portions of the polymer backbone that are regular but not repetitive are called.
Web the most common types of secondary structures are the α helix and the β pleated sheet. A helix with 2 residues/turn. The arrangement of each successive peptide plane is pleated due to the tetrahedral nature of the alpha. Both structures are held in shape by hydrogen bonds, which form between the carbonyl o of one amino acid and the. They both are shaped by hydrogen bonding between the carbonyl o of one amino acid and the amino h of another. The other portions of the polymer backbone that are regular but not repetitive are called.
Secondary structures of keratin protein (beta pleated sheets and alpha
A helix with 2 residues/turn. Both structures are held in shape by hydrogen bonds, which form between the carbonyl o of one amino acid and the. Web the most common types of secondary structures are the α helix and the β pleated sheet. They both are shaped by hydrogen bonding between the carbonyl o of one amino acid and the.
1. Secondary structure of protein, αhelix and βpleated sheet [118
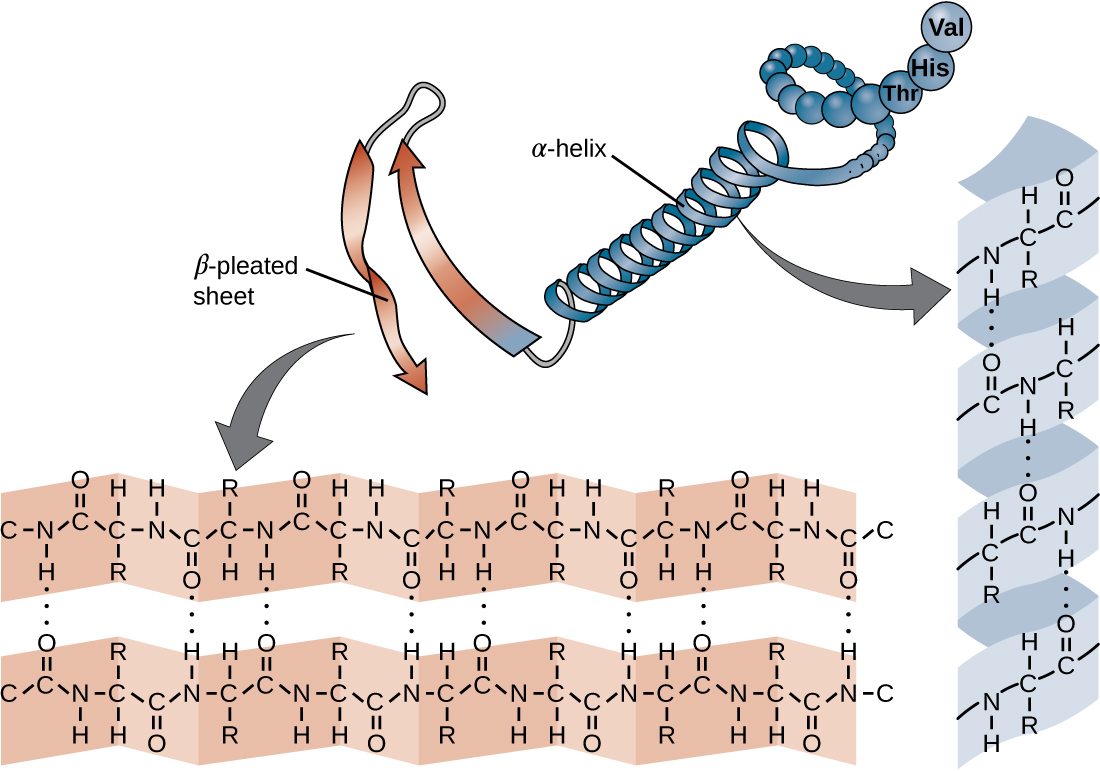
Web the most common types of secondary structures are the α helix and the β pleated sheet. They both are shaped by hydrogen bonding between the carbonyl o of one amino acid and the amino h of another. The other portions of the polymer backbone that are regular but not repetitive are called. The arrangement of each successive peptide plane.
Alpha Helix vs Beta Pleated Sheet Diffzi
A helix with 2 residues/turn. The arrangement of each successive peptide plane is pleated due to the tetrahedral nature of the alpha. They both are shaped by hydrogen bonding between the carbonyl o of one amino acid and the amino h of another. Web the most common types of secondary structures are the α helix and the β pleated sheet..
College. Science. Life Essential Cell Biology 3rd Ch 4 Protein
A helix with 2 residues/turn. Both structures are held in shape by hydrogen bonds, which form between the carbonyl o of one amino acid and the. They both are shaped by hydrogen bonding between the carbonyl o of one amino acid and the amino h of another. The other portions of the polymer backbone that are regular but not repetitive.
Difference Between Alpha Helix and Beta Pleated Sheet infographic
Both structures are held in shape by hydrogen bonds, which form between the carbonyl o of one amino acid and the. They both are shaped by hydrogen bonding between the carbonyl o of one amino acid and the amino h of another. The other portions of the polymer backbone that are regular but not repetitive are called. The arrangement of.
The Difference Between Alpha And Beta Sheets
A helix with 2 residues/turn. The arrangement of each successive peptide plane is pleated due to the tetrahedral nature of the alpha. They both are shaped by hydrogen bonding between the carbonyl o of one amino acid and the amino h of another. Web the most common types of secondary structures are the α helix and the β pleated sheet..
7.4 Proteins Biology LibreTexts
A helix with 2 residues/turn. The other portions of the polymer backbone that are regular but not repetitive are called. They both are shaped by hydrogen bonding between the carbonyl o of one amino acid and the amino h of another. Both structures are held in shape by hydrogen bonds, which form between the carbonyl o of one amino acid.
Alpha Helix Vs Beta Pleated Sheet What's The Difference? » Differencess
The arrangement of each successive peptide plane is pleated due to the tetrahedral nature of the alpha. Both structures are held in shape by hydrogen bonds, which form between the carbonyl o of one amino acid and the. Web the most common types of secondary structures are the α helix and the β pleated sheet. The other portions of the.
LabXchange
They both are shaped by hydrogen bonding between the carbonyl o of one amino acid and the amino h of another. A helix with 2 residues/turn. Web the most common types of secondary structures are the α helix and the β pleated sheet. The arrangement of each successive peptide plane is pleated due to the tetrahedral nature of the alpha..
Difference Between Alpha Helix and Beta Pleated Sheet Hydrogen Bond
They both are shaped by hydrogen bonding between the carbonyl o of one amino acid and the amino h of another. The other portions of the polymer backbone that are regular but not repetitive are called. A helix with 2 residues/turn. Both structures are held in shape by hydrogen bonds, which form between the carbonyl o of one amino acid.
Both Structures Are Held In Shape By Hydrogen Bonds, Which Form Between The Carbonyl O Of One Amino Acid And The.
Web the most common types of secondary structures are the α helix and the β pleated sheet. They both are shaped by hydrogen bonding between the carbonyl o of one amino acid and the amino h of another. A helix with 2 residues/turn. The arrangement of each successive peptide plane is pleated due to the tetrahedral nature of the alpha.