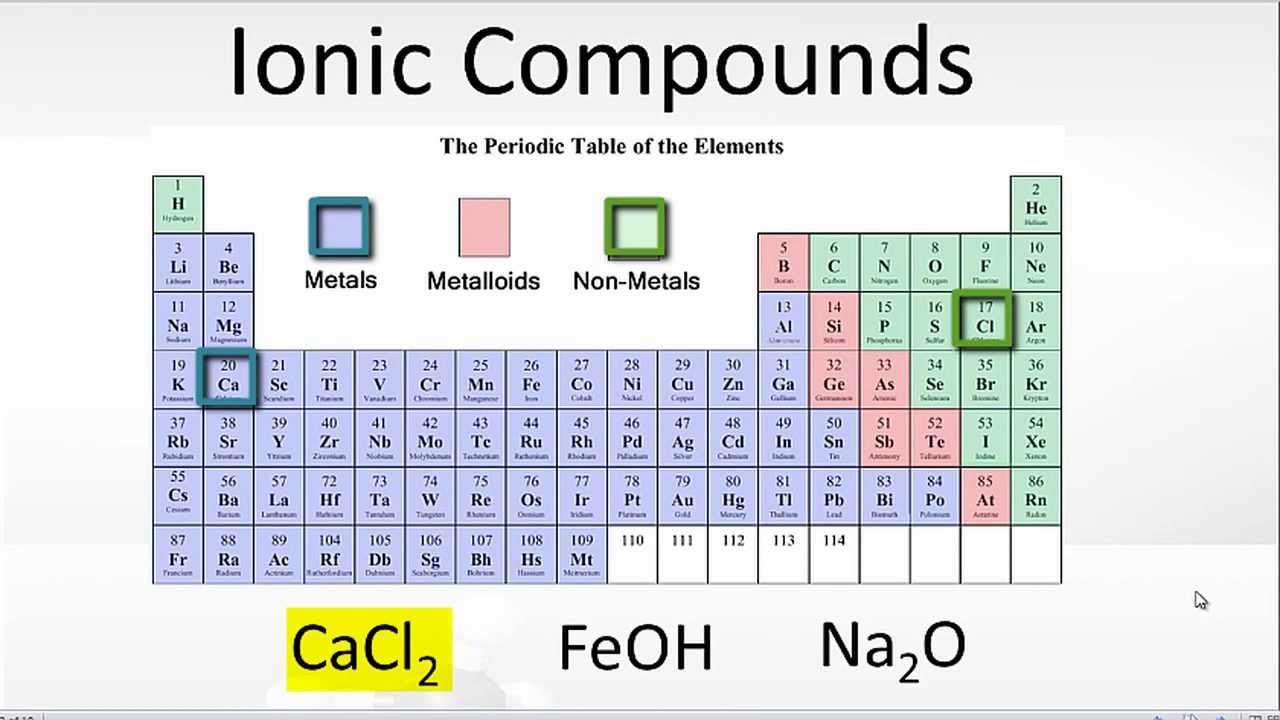
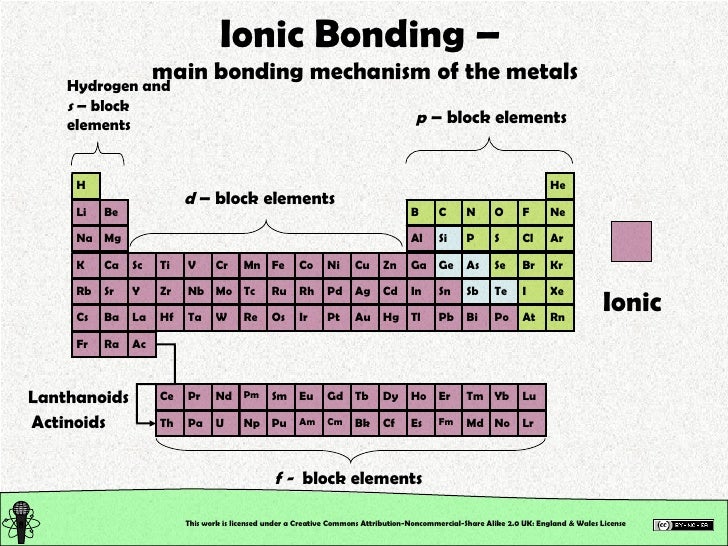
What Type Of Elements Form Ionic Bonds With Metals
What Type Of Elements Form Ionic Bonds With Metals - So how do you know what kind of bond an atom will make? Metals on the left and in the center of the periodic table form ionic bonds with nonmetals on the right of the. Web stephen lower simon fraser university learning objectives explain the fundamental difference between the bonding in metallic solids compared to that in other. Web ionic bonds form when two or more ions come together and are held together by charge differences. Web forming an ionic bond. Predict what other elements might form ionic bonds. Web ionic bonding occurs in compounds composed of strongly electropositive elements (metals) and strongly electronegative elements (nonmetals). Web ionic bonds usually occur between metal and nonmetal ions. These are electronegative elements with high ionization energies. Electrical conductivity how are ionic bonds formed?
This exchange results in a more stable, noble gas. Web stephen lower simon fraser university learning objectives explain the fundamental difference between the bonding in metallic solids compared to that in other. Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. The alkali halides (nacl, lif, etc.). Web forming an ionic bond. Predict what other elements might form ionic bonds. It is a type of chemical bond that generates two oppositely charged ions. The chemical bond that is formed between 2 2 atoms through the transfer of one or more electrons from the electropositive or metallic element. Electrical conductivity how are ionic bonds formed? So how do you know what kind of bond an atom will make?
Web ionic bonds are one of the two main types of chemical bonds. Web ionic bonding is the complete transfer of valence electron (s) between atoms. Boiling and melting point 4. The alkali halides (nacl, lif, etc.). Web ionic bonds form when two or more ions come together and are held together by charge differences. Web ionic bonds usually occur between metal and nonmetal ions. This exchange results in a more stable, noble gas. These are electronegative elements with high ionization energies. Electrical conductivity how are ionic bonds formed? Web the three ions would adhere (bond) to each other by the positive/negative attraction between the ions.
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures
Web ionic bonding is the complete transfer of valence electron (s) between atoms. Web ionic bonding occurs in compounds composed of strongly electropositive elements (metals) and strongly electronegative elements (nonmetals). The alkali halides (nacl, lif, etc.). Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. Metals on the.
Ionic Properties
Web forming an ionic bond. Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. Web ionic bonding is the complete transfer of valence electron (s) between atoms. They form as a result of electrostatic attraction between oppositely charged ions and usually occur. Web metals and nonmetals are involved.
Ionic Bond Definition, Types, Properties & Examples
These are electronegative elements with high ionization energies. Web ionic bonding occurs in compounds composed of strongly electropositive elements (metals) and strongly electronegative elements (nonmetals). This exchange results in a more stable, noble gas. Web ionic bonds are one of the two main types of chemical bonds. The alkali halides (nacl, lif, etc.).
Ionic Bond Definition Easy Hard Science
Predict what other elements might form ionic bonds. For example, sodium (na), a metal, and chloride (cl), a nonmetal, form an ionic bond to make nacl. This exchange results in a more stable, noble gas. Web the three ions would adhere (bond) to each other by the positive/negative attraction between the ions. Metals on the left and in the center.
Naming Simple Ionic Compounds Pathways to Chemistry
Metals on the left and in the center of the periodic table form ionic bonds with nonmetals on the right of the. Web ionic bonds usually occur between metal and nonmetal ions. Web the three ions would adhere (bond) to each other by the positive/negative attraction between the ions. Web stephen lower simon fraser university learning objectives explain the fundamental.
Ionic Bond Definition, Types, Properties & Examples
The alkali halides (nacl, lif, etc.). Boiling and melting point 4. Web the three ions would adhere (bond) to each other by the positive/negative attraction between the ions. For example, sodium (na), a metal, and chloride (cl), a nonmetal, form an ionic bond to make nacl. Electrical conductivity how are ionic bonds formed?
2.6 Ionic Compounds and Formulas Chemistry LibreTexts
Web ionic bonding is the complete transfer of valence electron (s) between atoms. So how do you know what kind of bond an atom will make? Boiling and melting point 4. The chemical bond that is formed between 2 2 atoms through the transfer of one or more electrons from the electropositive or metallic element. Web the three ions would.
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare
Web ionic bonds form when two or more ions come together and are held together by charge differences. Web the three ions would adhere (bond) to each other by the positive/negative attraction between the ions. It is a type of chemical bond that generates two oppositely charged ions. Ionic bonding is a type of chemical bond in which valence electrons.
Periodic Table Ions List Periodic Table Timeline
An atom of sodium will lose an electron and form a positive ion. Web metals and nonmetals are involved in ionic bonding, but it specifically refers to two elements that are oppositely charged being bonded to eachother. The alkali halides (nacl, lif, etc.). Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom.
Chemical Structure Chemical Bonding. Ionic, Metallic & Coordinate Bo…
Web ionic bonds form when two or more ions come together and are held together by charge differences. Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another. Web the three ions would adhere (bond) to each other by the positive/negative attraction between the ions. Web ionic bonds are.
Web Metals And Nonmetals Are Involved In Ionic Bonding, But It Specifically Refers To Two Elements That Are Oppositely Charged Being Bonded To Eachother.
For example, sodium (na), a metal, and chloride (cl), a nonmetal, form an ionic bond to make nacl. Web ionic bonding occurs in compounds composed of strongly electropositive elements (metals) and strongly electronegative elements (nonmetals). Web ionic bonds are one of the two main types of chemical bonds. These are electronegative elements with high ionization energies.
It Is A Type Of Chemical Bond That Generates Two Oppositely Charged Ions.
They form as a result of electrostatic attraction between oppositely charged ions and usually occur. Electrical conductivity how are ionic bonds formed? Predict what other elements might form ionic bonds. The chemical bond that is formed between 2 2 atoms through the transfer of one or more electrons from the electropositive or metallic element.
Web Stephen Lower Simon Fraser University Learning Objectives Explain The Fundamental Difference Between The Bonding In Metallic Solids Compared To That In Other.
Boiling and melting point 4. Web ionic bonding is the complete transfer of valence electron (s) between atoms. Web forming an ionic bond. Ionic bonding is a type of chemical bond in which valence electrons are lost from one atom and gained by another.
The Alkali Halides (Nacl, Lif, Etc.).
So how do you know what kind of bond an atom will make? Web the three ions would adhere (bond) to each other by the positive/negative attraction between the ions. An atom of sodium will lose an electron and form a positive ion. This exchange results in a more stable, noble gas.



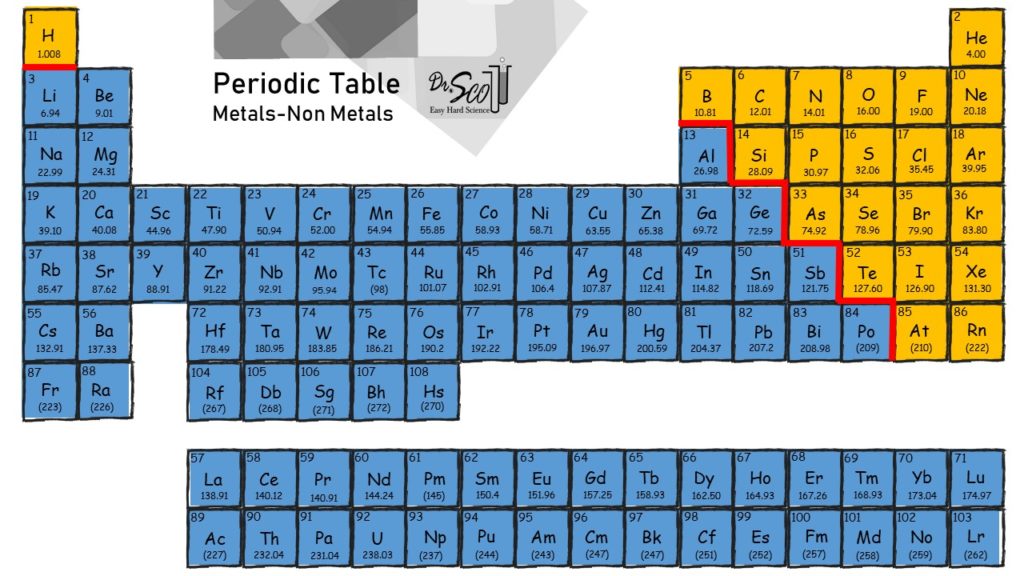


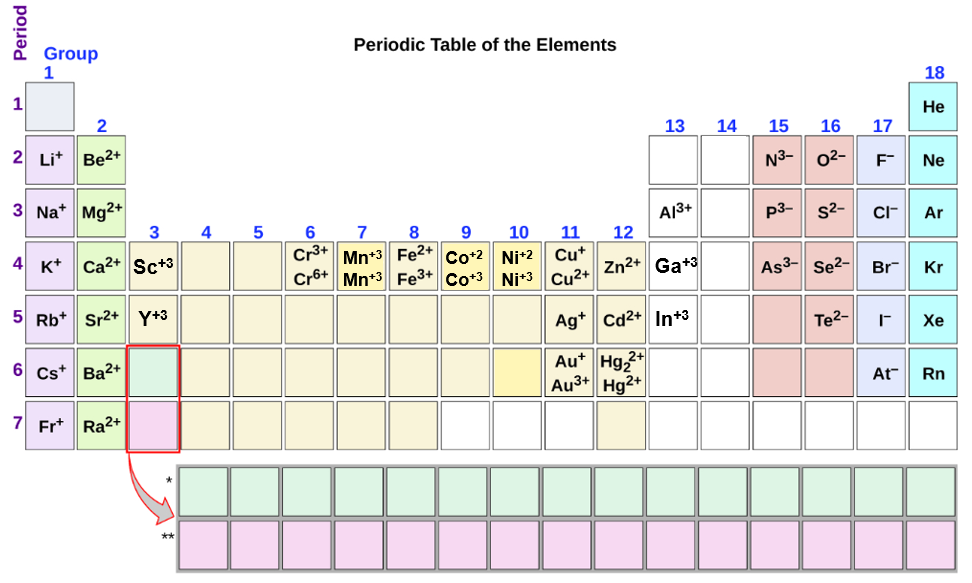
.PNG)