Which Elements Tend To Form Covalent Bonds
Which Elements Tend To Form Covalent Bonds - Web atoms share electrons b. Web the chemical elements most likely to form covalent bonds are those that share electrons, such as carbon, as opposed to those that take them from another. Group iiib, ivb, vb, vib, viib, viiib,. Two elements each looking to gain an electron or electrons. How many electrons are shared in a double. Share a pair of electrons. Web how many electrons are shared in a double covalent bond? Web there are two types of covalent bonds: Web terms in this set (110) ion. H 2o2 this is what.
Web covalent bonds form between atoms of nonmetallic elements. Web covalent bonds are named based on their nature. An atom that has gained or lost one or more electrons. In general, bonds are considered to be covalent if the electronegativity difference between the two. Group iiib, ivb, vb, vib, viib, viiib,. Positively charged and negatively charged parts of covalent molecules attract c. The term covalent bond is used to. Web molecular shape isomerism in organic compounds there are many types of chemical bonds and forces that bind molecules together. Web there are two types of covalent bonds: Web predict the number of covalent bonds formed based on the elements involved and their position on the periodic table.
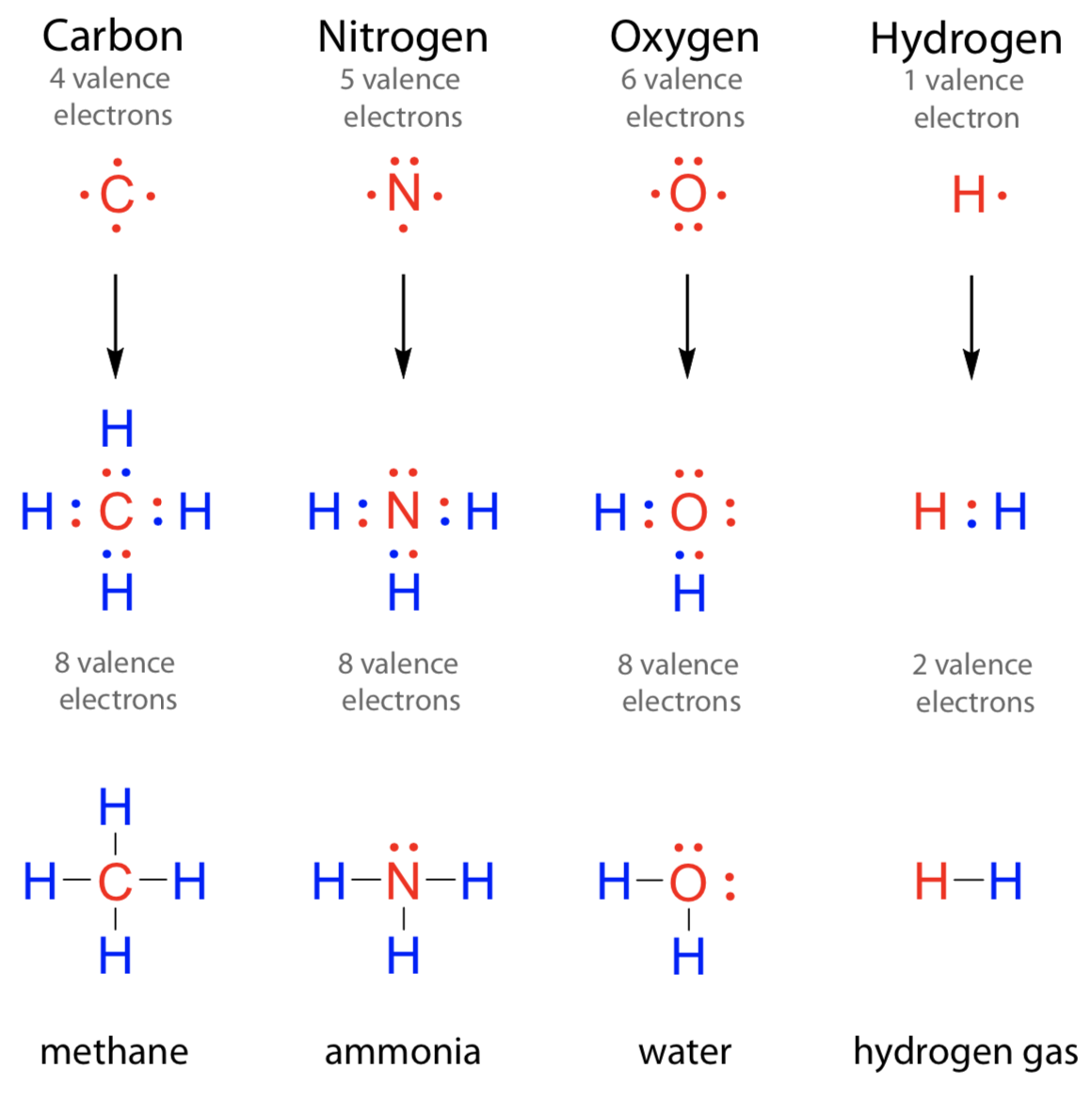
Two elements each looking to gain an electron or electrons. Web terms in this set (110) ion. Elements don't like to have unpaired electrons so they either want to get rid of the extra. H 2o2 this is what. Web answer (1 of 25): Web molecular shape isomerism in organic compounds there are many types of chemical bonds and forces that bind molecules together. The two most basic types of. Web a pair of oxygen atoms can form an o 2 molecule in which each atom has a total of eight valence electrons by sharing two pairs of electrons. Web how many electrons are shared in a double covalent bond? How many electrons are shared in a double.
LabXchange
The electrons involved are in the outer shells of the atoms. Web covalent bonds are named based on their nature. Elements don't like to have unpaired electrons so they either want to get rid of the extra. Group iiib, ivb, vb, vib, viib, viiib,. How many electrons are shared in a double.
Explain what a covalent bond is, what types of elements form covalent
Web which elements tend to form covalent bonds? In general, bonds are considered to be covalent if the electronegativity difference between the two. Covalency occurs when 2 or more atoms share their electrons so that they get they get their octet of electrons. Web answer (1 of 25): The two most basic types of.
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
H 2o2 this is what. The number of bonds an element forms in a covalent compound is determined by the. Ions with opposite electrical charges attract d. Web covalent bonds form between atoms of nonmetallic elements. Web predict the number of covalent bonds formed based on the elements involved and their position on the periodic table.
Covalent Bond Biology Dictionary
H 2o2 this is what. Web a covalent bond is formed between two atoms by sharing electrons. Web atoms share electrons b. Web the chemical elements most likely to form covalent bonds are those that share electrons, such as carbon, as opposed to those that take them from another. I don’t know if metalloids also do that or not, though.
Covalent Bonding
Nonpolar covalent bonds form between two atoms of the same element or between different elements that share. Ions with opposite electrical charges attract d. Web how many electrons are shared in a double covalent bond? Web which elements tend to form covalent bonds? Web a covalent bond is formed between two atoms by sharing electrons.
Covalent Bonds The Basics of Chemical Bonding
Web molecular shape isomerism in organic compounds there are many types of chemical bonds and forces that bind molecules together. Web a covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. The two most basic types of. Web covalent bonds form between atoms of nonmetallic elements. These electron pairs are known.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Describe the important exceptions to the. Elements don't like to have unpaired electrons so they either want to get rid of the extra. Nonpolar covalent bonds form between two atoms of the same element or between different elements that share. Web a covalent bond is formed between two atoms by sharing electrons. H 2o2 this is what.
Forms of Binding in Crystals Overall Science
Web a covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. I don’t know if metalloids also do that or not, though. Web which elements tend to form covalent bonds? Web molecular shape isomerism in organic compounds there are many types of chemical bonds and forces that bind molecules together. How.
Which elements form a covalent bond? Quora
Web a covalent bond is formed between two atoms by sharing electrons. These electron pairs are known as shared pairs or bonding pairs. Web how many electrons are shared in a double covalent bond? Web atoms share electrons b. Web molecular shape isomerism in organic compounds there are many types of chemical bonds and forces that bind molecules together.
Web A Pair Of Oxygen Atoms Can Form An O 2 Molecule In Which Each Atom Has A Total Of Eight Valence Electrons By Sharing Two Pairs Of Electrons.
I don’t know if metalloids also do that or not, though. Web covalent bonds form between atoms of nonmetallic elements. In general, bonds are considered to be covalent if the electronegativity difference between the two. Web atoms share electrons b.
Share A Pair Of Electrons.
The electrons involved are in the outer shells of the atoms. The number of bonds an element forms in a covalent compound is determined by the. Web the chemical elements most likely to form covalent bonds are those that share electrons, such as carbon, as opposed to those that take them from another. Web how many electrons are shared in a double covalent bond?
An Atom That Has Gained Or Lost One Or More Electrons.
Covalency occurs when 2 or more atoms share their electrons so that they get they get their octet of electrons. Nonpolar covalent bonds form between two atoms of the same element or between different elements that share. Web a covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. Web terms in this set (110) ion.
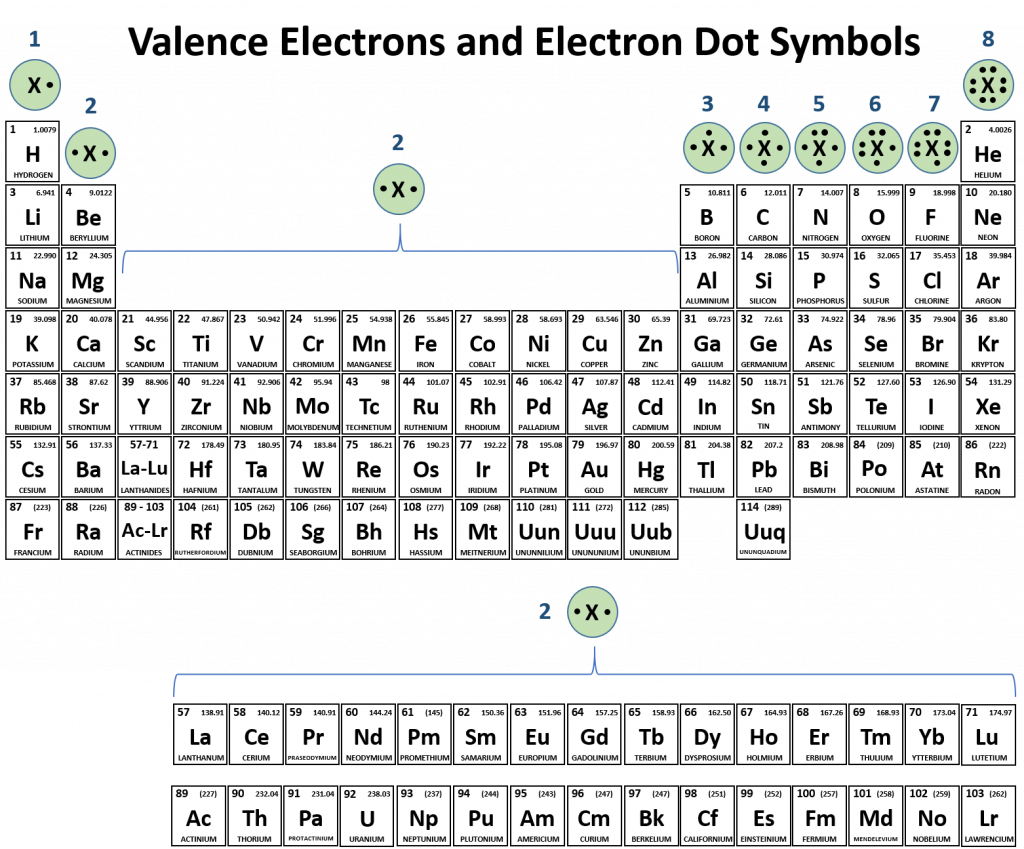
Web Predict The Number Of Covalent Bonds Formed Based On The Elements Involved And Their Position On The Periodic Table.
The term covalent bond is used to. Web answer (1 of 25): These electron pairs are known as shared pairs or bonding pairs. How many electrons are shared in a double.