Which Two Elements Would Form A Covalent Compound
Which Two Elements Would Form A Covalent Compound - By sharing an electron they satisfy the octet rule for both atoms. To apply the octet rule to covalent compounds you have already seen examples of substances that. A table of lewis dot. Web the two atoms which have low electronegativity difference that is they are non metals can form a covalent bond. Web a covalent bond forming h 2 (right) where two hydrogen atoms share the two electrons. Web covalent bonding is the sharing of electrons between atoms. The electrons involved are in the outer shells of the atoms. This type of bonding occurs between two atoms of the same element or of elements close to each. We refer to this as a. The element carbon (c) is most likely to form covalent bonds with the element.
The element carbon (c) is most likely to form covalent bonds with the element. Web learning objectives to describe how a covalent bond forms. Web each atom can share two electrons with the other so that each atom has eight valence electrons. Web the two atoms which have low electronegativity difference that is they are non metals can form a covalent bond. We refer to this as a. To apply the octet rule to covalent compounds you have already seen examples of substances that. A covalent bond is a chemical bond that involves the sharing of electrons to form electron. A table of lewis dot. Web when the electronegativities of the elements in a compound are about the same, the atoms share electrons, and the substance is covalent. Web a covalent bond forming h 2 (right) where two hydrogen atoms share the two electrons.
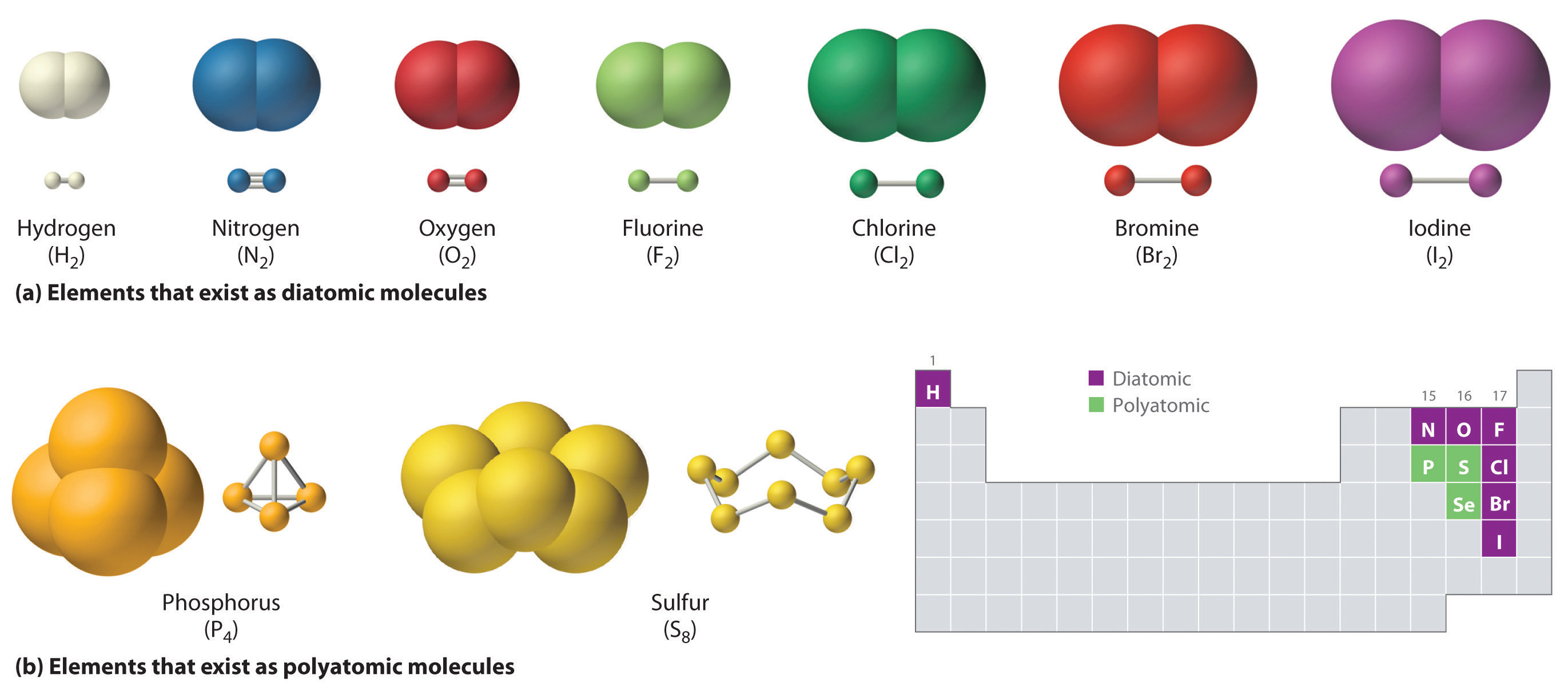
A table of lewis dot. For example, the hydrogen molecule, h 2, contains a covalent bond between its two hydrogen atoms. Web hydrogen, oxygen, nitrogen and the halogens all form these types of bonds. Covalent bond is defined as a type of bond which is. We refer to this as a. The element carbon (c) is most likely to form covalent bonds with the element. Containing covalent bonds between two of the same type of atom are only a few. Is formed when two atoms. Web the two atoms which have low electronegativity difference that is they are non metals can form a covalent bond. A covalent bond is a chemical bond that involves the sharing of electrons to form electron.
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
Web it takes two electrons to make a covalent bond, one from each bonding atom. Web covalent and ionic compounds. The electrons involved are in the outer shells of the atoms. Containing covalent bonds between two of the same type of atom are only a few. Web covalent compounds are formed when the electronegativity values of the elements in a.
Covalent Bond Biology Dictionary
What elements make covalent bonds? Web nonmetal atoms frequently form covalent bonds with other nonmetal atoms. To appreciate how atoms share their valence electrons in covalent. As a general rule, atoms form covalent. Web if the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules, then the electrons in the bond.
Covalent Bond Examples Several Examples of Covalent (molecular) Bonds
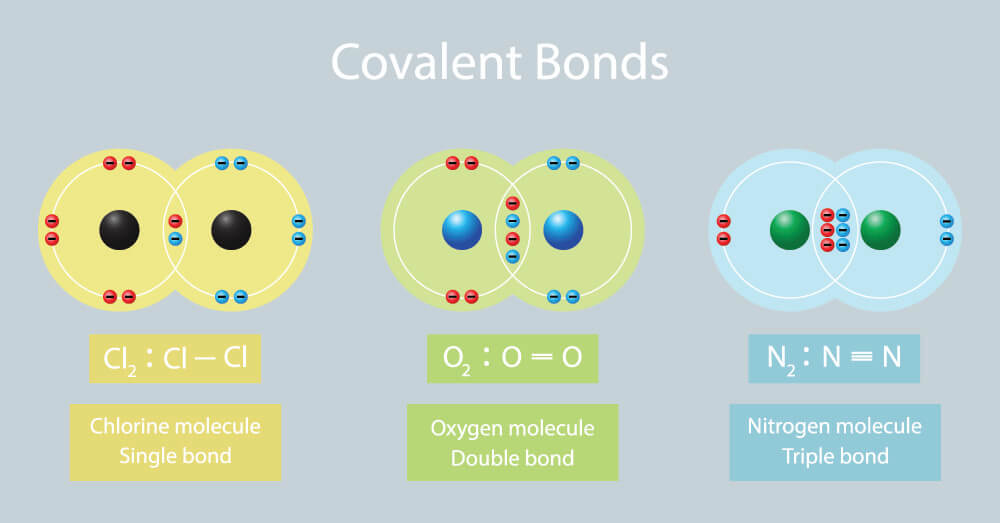
Share a pair of electrons. Lewis dot structures are one way to represent how atoms form covalent bonds. Web if the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules, then the electrons in the bond must be shared equally. To apply the octet rule to covalent compounds you have already.
What Is a Covalent Compound?
To apply the octet rule to covalent compounds you have already seen examples of substances that. Is formed when two atoms. Covalent bond is defined as a type of bond which is. Covalent bonds form when two or more nonmetals combine. The electrons involved are in the outer shells of the atoms.
Covalent Bonds Biology for NonMajors I
Web it takes two electrons to make a covalent bond, one from each bonding atom. For example, the hydrogen molecule, h 2, contains a covalent bond between its two hydrogen atoms. As a general rule, atoms form covalent. The electrons involved are in the outer shells of the atoms. By sharing an electron they satisfy the octet rule for both.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Web nonmetal atoms frequently form covalent bonds with other nonmetal atoms. To appreciate how atoms share their valence electrons in covalent. By sharing an electron they satisfy the octet rule for both atoms. Web learning objectives to describe how a covalent bond forms. The element carbon (c) is most likely to form covalent bonds with the element.
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
Is formed when two atoms. Web each atom can share two electrons with the other so that each atom has eight valence electrons. By sharing an electron they satisfy the octet rule for both atoms. Share a pair of electrons. Web a covalent bond forming h 2 (right) where two hydrogen atoms share the two electrons.
CH150 Chapter 3 Ions and Ionic Compounds Chemistry
For example, the hydrogen molecule, h 2, contains a covalent bond between its two hydrogen atoms. Web each atom can share two electrons with the other so that each atom has eight valence electrons. Web hydrogen, oxygen, nitrogen and the halogens all form these types of bonds. Web diatomic molecules such as hydrogen ( h 2 ), chlorine ( cl.
コンパウンド Compound JapaneseClass.jp
Web learning objectives to know what types of elements bond to form covalent compounds. Web covalent and ionic compounds. Share a pair of electrons. A table of lewis dot. Web hydrogen, oxygen, nitrogen and the halogens all form these types of bonds.
Covalent Bonding (Biology) — Definition & Role Expii
Web hydrogen, oxygen, nitrogen and the halogens all form these types of bonds. To appreciate how atoms share their valence electrons in covalent. Containing covalent bonds between two of the same type of atom are only a few. Share a pair of electrons. To apply the octet rule to covalent compounds you have already seen examples of substances that.
Web It Takes Two Electrons To Make A Covalent Bond, One From Each Bonding Atom.
Containing covalent bonds between two of the same type of atom are only a few. Web diatomic molecules such as hydrogen ( h 2 ), chlorine ( cl 2 ), fluorine ( f 2 ), etc. To apply the octet rule to covalent compounds you have already seen examples of substances that. As a general rule, atoms form covalent.
Is Formed When Two Atoms.
Web if the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules, then the electrons in the bond must be shared equally. Web learning objectives to describe how a covalent bond forms. Share a pair of electrons. Web covalent bonding is the sharing of electrons between atoms.
To Appreciate How Atoms Share Their Valence Electrons In Covalent.
Web nonmetal atoms frequently form covalent bonds with other nonmetal atoms. Web learning objectives to know what types of elements bond to form covalent compounds. For example, the hydrogen molecule, h 2, contains a covalent bond between its two hydrogen atoms. Web covalent compounds are formed when the electronegativity values of the elements in a compound are identical or similar.
Web Each Atom Can Share Two Electrons With The Other So That Each Atom Has Eight Valence Electrons.
A covalent bond is a chemical bond that involves the sharing of electrons to form electron. Covalent bonds form when two or more nonmetals combine. Web a covalent bond forming h 2 (right) where two hydrogen atoms share the two electrons. Web hydrogen, oxygen, nitrogen and the halogens all form these types of bonds.


:max_bytes(150000):strip_icc()/water-56a128af5f9b58b7d0bc93c3.jpg)