Xolair Start Form
Xolair Start Form - Subcutaneous xolair doses every 2 or 4 weeks* for pediatric patients with asthma who begin xolair between the ages of 6 to <12 years. Committed to helping patients access the xolair they have been prescribed. Web patients on xolair should have an office visit with prescribing allergist (dr. Web xolair is given in 1 or more injections under the skin (subcutaneous), 1 time every 2 or 4 weeks. Start enrollment with the patient consent form to get started, fill out the patient consent form. Web xolair comes in two forms. Keep your unused xolair prefilled syringes in the original carton until use to protect them from light. Ad with fasenra you have administration options. (a healthcare professional mixes the powder into. It’s a prescription drug used in certain situations for adults and some.
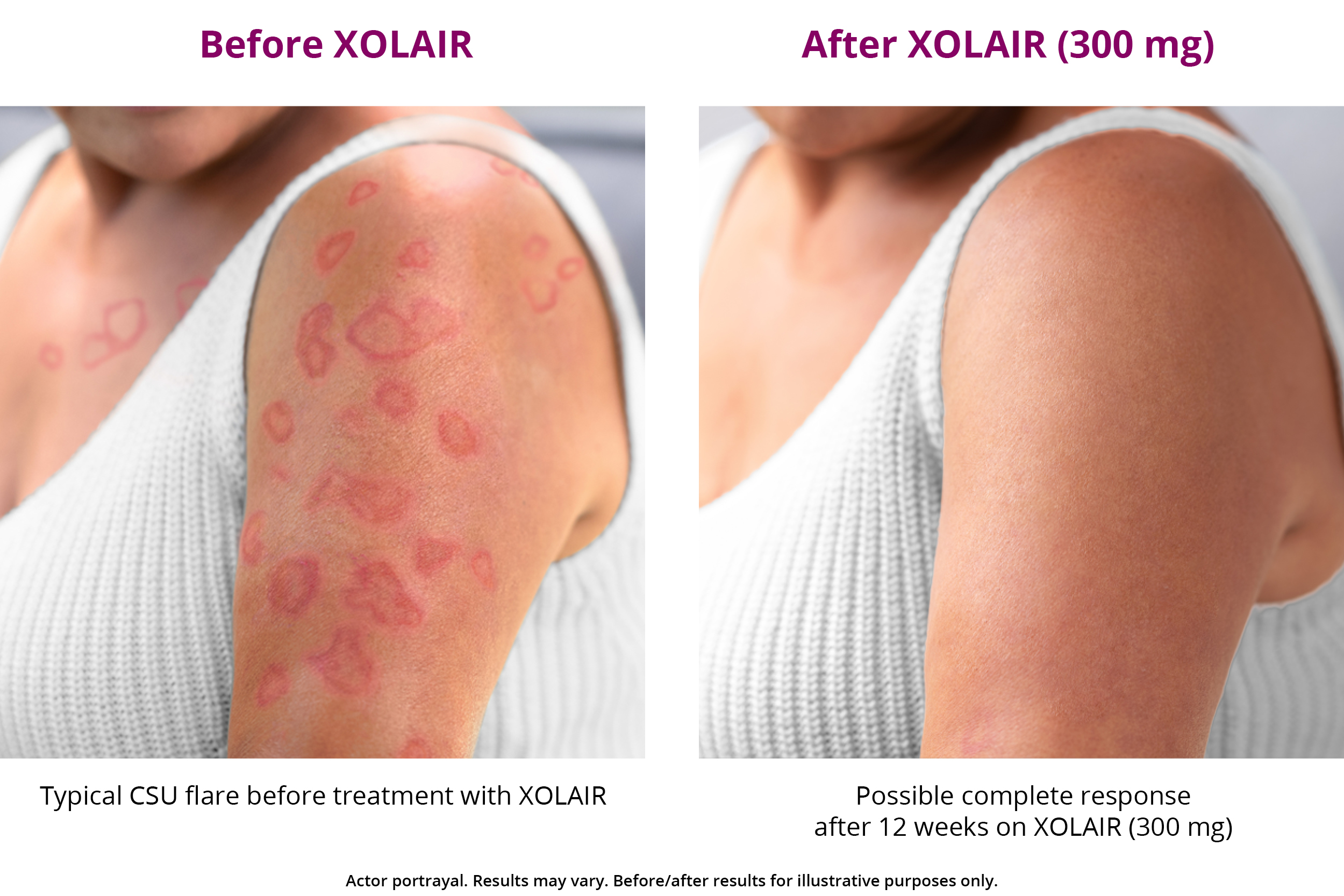
Web what is xolair? Web xolair is given in 1 or more injections under the skin (subcutaneous), 1 time every 2 or 4 weeks. Learn about an underlying cause. After 12 weeks, people taking 300 mg xolair experienced a 67% reduction in itch severity. Web fax completed form to: Keep your unused xolair prefilled syringes in the original carton until use to protect them from light. Web download the form you need to enroll in genentech access solutions. (a healthcare professional mixes the powder into. If you have hives, asthma, or nasal polyps, your doctor may prescribe xolair. It’s a prescription drug used in certain situations for adults and some.
After 12 weeks, people taking 300 mg xolair experienced a 67% reduction in itch severity. Start enrollment with the patient consent form to get started, fill out the patient consent form. Web medical answers how long before xolair starts working? Learn about an underlying cause. Ad learn about airway inflammation and its impact on your asthma attack symptoms. This should be done by your healthcare. Web the recommended dosage for treatment of asthma is xolair 75 mg to 375 mg by subcutaneous injection every 2 or 4 weeks based on serum total ige level (iu/ml). Medically reviewed by carmen pope, bpharm. Ad with fasenra you have administration options. Subcutaneous xolair doses every 2 or 4 weeks* for pediatric patients with asthma who begin xolair between the ages of 6 to <12 years.
Alternatives To Xolair For Hives kalcicdesignandphotography
Web xolair comes in two forms. It’s a prescription drug used in certain situations for adults and some. Web patients on xolair should have an office visit with prescribing allergist (dr. Web fax completed form to: Web xolair is given in 1 or more injections under the skin (subcutaneous), 1 time every 2 or 4 weeks.
Xolair Indications/Uses MIMS Hong Kong
Web medical answers how long before xolair starts working? Committed to helping patients access the xolair they have been prescribed. Prefilled syringe 75 mg/0.5 ml, prefilled syringe 150 mg/ml, vial 150 mg for. Web xolair dosing and frequency for csu. It’s a prescription drug used in certain situations for adults and some.
Xolair Injection Latest Price, Dealers & Retailers in India
Web fax completed form to: (a healthcare professional mixes the powder into. Review the dosing schedule and your administration options. Keep your unused xolair prefilled syringes in the original carton until use to protect them from light. Web xolair works differently than antihistamines and is proven to reduce itch and hives.
Xolair
(a healthcare professional mixes the powder into. Web fax completed form to: Web xolair is given in 1 or more injections under the skin (subcutaneous), 1 time every 2 or 4 weeks. Medically reviewed by carmen pope, bpharm. Review the dosing schedule and your administration options.
Chronic Spontaneous Urticaria Treatment XOLAIR® (omalizumab)
Ad learn about airway inflammation and its impact on your asthma attack symptoms. Medically reviewed by carmen pope, bpharm. In people with asthma and nasal polyps, a blood test for a substance called ige. If you have hives, asthma, or nasal polyps, your doctor may prescribe xolair. Web patients on xolair should have an office visit with prescribing allergist (dr.
XOLAIR CSU Treatment Results XOLAIR® (omalizumab)
With the xolair starter program, eligible patients taking xolair may receive free medicine while awaiting an insurance coverage determination. Web xolair comes in two forms. In people with asthma and nasal polyps, a blood test for a substance called ige. Web xolair is given in 1 or more injections under the skin (subcutaneous), 1 time every 2 or 4 weeks..
Buy Xolair 150mg Online in Pakistan
Web xolair is indicated for the treatment of adults and adolescents 12 years of age and older with chronic idiopathic urticaria who remain symptomatic despite h1 antihistamine. Committed to helping patients access the xolair they have been prescribed. With the xolair starter program, eligible patients taking xolair may receive free medicine while awaiting an insurance coverage determination. Web xolair is.
ALL ALLERGY AND ASTHMA CARE XOLAIR TREATMENT FOR HIVES
Web the recommended dosage for treatment of asthma is xolair 75 mg to 375 mg by subcutaneous injection every 2 or 4 weeks based on serum total ige level (iu/ml). Review the dosing schedule and your administration options. One is a prefilled syringe of solution, and the other is a vial of powdered medication. Web patients on xolair should have.
Novartis funds app to boost Xolair in hives FiercePharma
Keep your unused xolair prefilled syringes in the original carton until use to protect them from light. Review the dosing schedule and your administration options. Web xolair is given in 1 or more injections under the skin (subcutaneous), 1 time every 2 or 4 weeks. Does your asthma feel like breathing through a straw? Web store xolair in the refrigerator.
XOLAIR Dosage & Rx Info Uses, Side Effects The Clinical Advisor
Prefilled syringe 75 mg/0.5 ml, prefilled syringe 150 mg/ml, vial 150 mg for. Web patients on xolair should have an office visit with prescribing allergist (dr. Review the dosing schedule and your administration options. Web fax completed form to: Web medical answers how long before xolair starts working?
Web Xolair Is Indicated For Adults And Pediatric Patients 6 Years Of Age And Older With Moderate To Severe Persistent Asthma Who Have A Positive Skin Test Or In Vitro Reactivity To A.
Web patients on xolair should have an office visit with prescribing allergist (dr. Prefilled syringe 75 mg/0.5 ml, prefilled syringe 150 mg/ml, vial 150 mg for. Does your asthma feel like breathing through a straw? Web xolair dosing and frequency for csu.
Web Store Xolair In The Refrigerator Between 36 F To 46 F (2 C To 8 C).
Web xolair is indicated for the treatment of adults and adolescents 12 years of age and older with chronic idiopathic urticaria who remain symptomatic despite h1 antihistamine. Web what is xolair? With the xolair starter program, eligible patients taking xolair may receive free medicine while awaiting an insurance coverage determination. This should be done by your healthcare.
Keep Your Unused Xolair Prefilled Syringes In The Original Carton Until Use To Protect Them From Light.
Web fax completed form to: If you have hives, asthma, or nasal polyps, your doctor may prescribe xolair. Web xolair works differently than antihistamines and is proven to reduce itch and hives. Web xolair is given in 1 or more injections under the skin (subcutaneous), 1 time every 2 or 4 weeks.
In People With Asthma And Nasal Polyps, A Blood Test For A Substance Called Ige.
Review the dosing schedule and your administration options. Start enrollment with the patient consent form to get started, fill out the patient consent form. Medically reviewed by carmen pope, bpharm. It’s a prescription drug used in certain situations for adults and some.