How Many Bonds Can Hydrogen Form
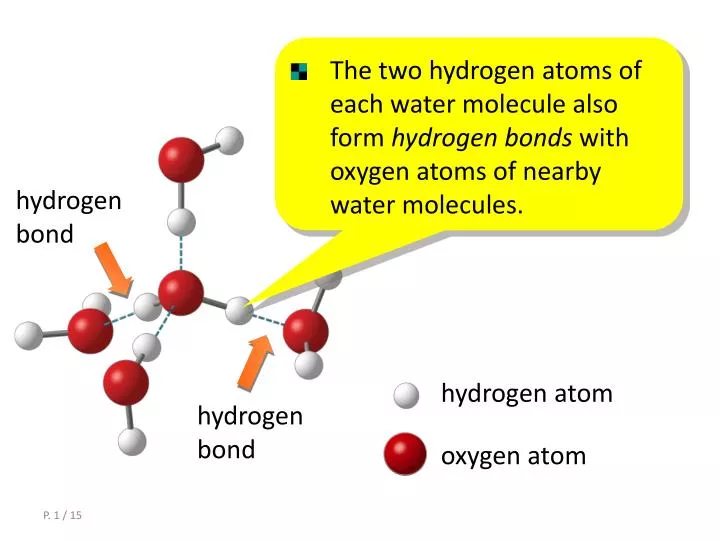
How Many Bonds Can Hydrogen Form - Web a single hydrogen atom can participate in two hydrogen bonds. Using pauling's scale—c (2.55) and h. Thus one hydrogen atom will only bond once. Web methane, ( ch 4, is a single carbon atom covalently bonded to four hydrogen atoms. It can exist, for instance, in complex. Web so far, we’ve drawn this water molecule with one hydrogen bond. Web any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. In these examples the central atoms form different numbers of bonds. Each selenium atom can form two bonds, one with each hydrogen (2 hydrogen atoms total). Two with the hydrogen atoms and two with the with.
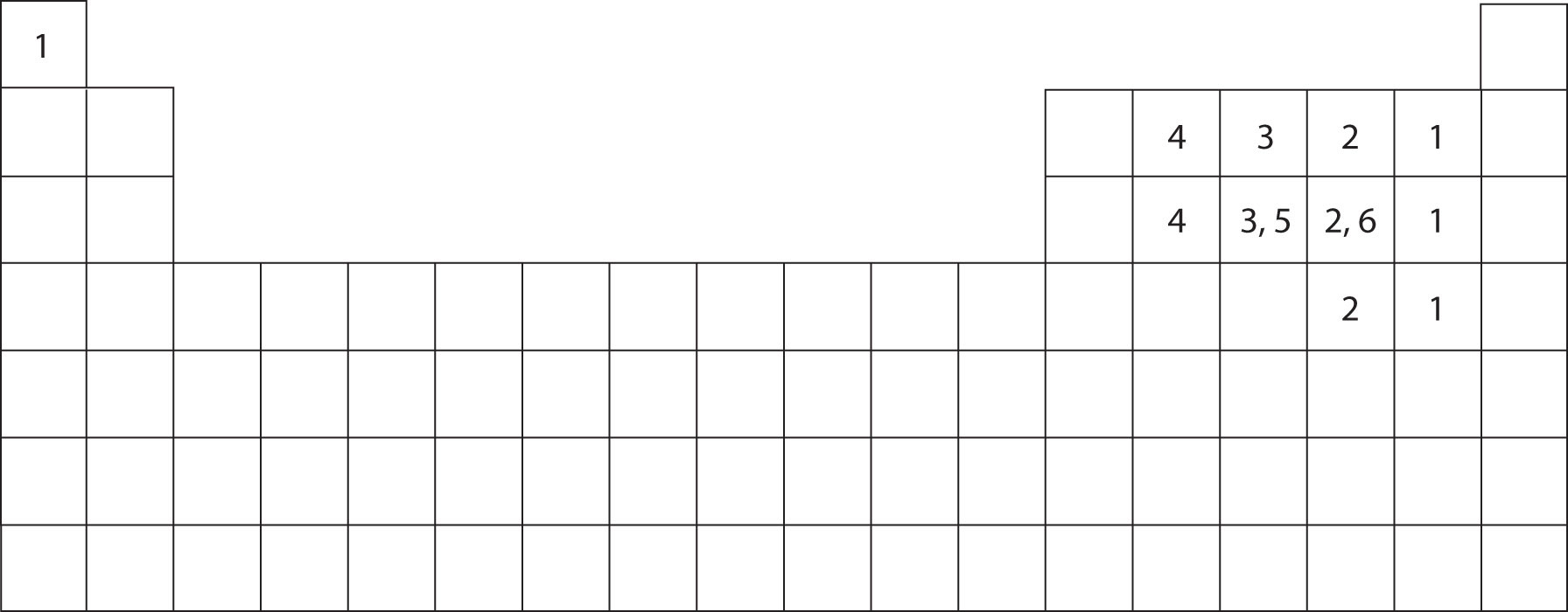
Web each hydrogen can form one bond with selenium. Web a single hydrogen atom can participate in two hydrogen bonds. Web the number refers to the number of bonds each of the element makes: Using pauling's scale—c (2.55) and h. Covalent bonds require pairs of electrons and hydrogen can only have two electrons bound in one. Such molecules will always have higher. Web apart from some group 13 weirdness, hydrogen can only make one bond. Web methane, ( ch 4, is a single carbon atom covalently bonded to four hydrogen atoms. Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules: It can exist, for instance, in complex.
Covalent bonds require pairs of electrons and hydrogen can only have two electrons bound in one. Such molecules will always have higher. Thus one hydrogen atom will only bond once. Another hydrogen bond can be formed using the other lone pair on the oxygen atom. Web since hydrogen has only one valence electron, it will only bond once. Web methane, ( ch 4, is a single carbon atom covalently bonded to four hydrogen atoms. Using pauling's scale—c (2.55) and h. Web the number refers to the number of bonds each of the element makes: Web so far, we’ve drawn this water molecule with one hydrogen bond. Hydrogen makes 1 bond, oxygen makes 2 bonds, nitrogen makes 3 bonds and.
Water
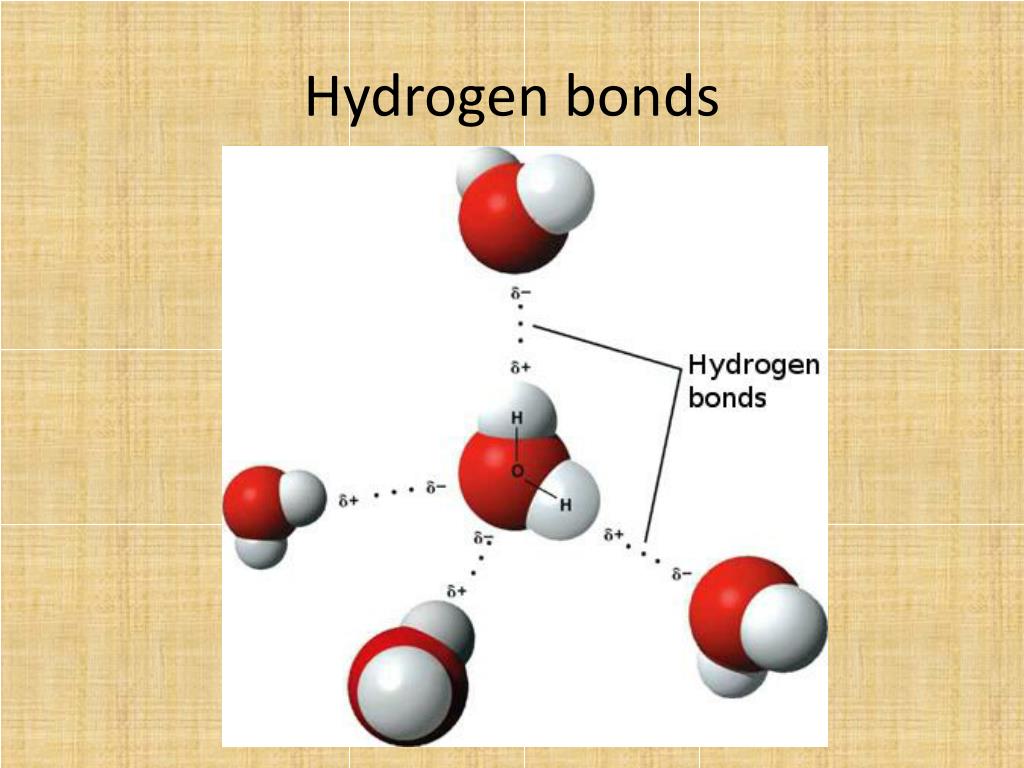
Web notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules: Such molecules will always have higher. Covalent bonds require pairs of electrons and hydrogen can only have two electrons bound in one. Web since hydrogen has only one valence electron, it will only bond once. Web each hydrogen can form one bond with selenium.
How many covalent bonds can hydrogen, oxygen, nitrogen and carbon form
Web a single hydrogen atom can participate in two hydrogen bonds. Two with the hydrogen atoms and two with the with. Hydrogen makes 1 bond, oxygen makes 2 bonds, nitrogen makes 3 bonds and. Using pauling's scale—c (2.55) and h. Web so far, we’ve drawn this water molecule with one hydrogen bond.
how many bonds does sulfur form
Using pauling's scale—c (2.55) and h. Another hydrogen bond can be formed using the other lone pair on the oxygen atom. Web the number refers to the number of bonds each of the element makes: Web each hydrogen can form one bond with selenium. Web any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen.
How many hydrogen bonds form by Nh3, H20 and HF and boiling point trend
Web any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding. Such molecules will always have higher. Web apart from some group 13 weirdness, hydrogen can only make one bond. Web methane, ( ch 4, is a single carbon atom covalently bonded to four hydrogen atoms. Thus one hydrogen atom.
PPT hydrogen bond PowerPoint Presentation, free download ID4524678
Such molecules will always have higher. Using pauling's scale—c (2.55) and h. Two with the hydrogen atoms and two with the with. Web since hydrogen has only one valence electron, it will only bond once. Another hydrogen bond can be formed using the other lone pair on the oxygen atom.
How many hydrogen bonds are attached to each water molecule in a solid
Each selenium atom can form two bonds, one with each hydrogen (2 hydrogen atoms total). Web since hydrogen has only one valence electron, it will only bond once. Web a single hydrogen atom can participate in two hydrogen bonds. Web so far, we’ve drawn this water molecule with one hydrogen bond. Another hydrogen bond can be formed using the other.
How many hydrogen bonds a water molecule can form Hydrogen Bonding in
Web so far, we’ve drawn this water molecule with one hydrogen bond. Web the number refers to the number of bonds each of the element makes: Web since hydrogen has only one valence electron, it will only bond once. Web methane, ( ch 4, is a single carbon atom covalently bonded to four hydrogen atoms. Such molecules will always have.
Hydrogen bonding Isaac's science blog
Web since hydrogen has only one valence electron, it will only bond once. Two with the hydrogen atoms and two with the with. Web the number refers to the number of bonds each of the element makes: Each selenium atom can form two bonds, one with each hydrogen (2 hydrogen atoms total). In these examples the central atoms form different.
HONC 1234 ChemSimplified
Thus one hydrogen atom will only bond once. Two with the hydrogen atoms and two with the with. Web since hydrogen has only one valence electron, it will only bond once. Web a single hydrogen atom can participate in two hydrogen bonds. Such molecules will always have higher.
PPT Chemistry of Life PowerPoint Presentation, free download ID2666943
Web a single hydrogen atom can participate in two hydrogen bonds. Thus one hydrogen atom will only bond once. Hydrogen makes 1 bond, oxygen makes 2 bonds, nitrogen makes 3 bonds and. Web since hydrogen has only one valence electron, it will only bond once. Web methane, ( ch 4, is a single carbon atom covalently bonded to four hydrogen.
Hydrogen Makes 1 Bond, Oxygen Makes 2 Bonds, Nitrogen Makes 3 Bonds And.
Web each hydrogen can form one bond with selenium. In these examples the central atoms form different numbers of bonds. Using pauling's scale—c (2.55) and h. Web any molecule which has a hydrogen atom attached directly to an oxygen or a nitrogen is capable of hydrogen bonding.
Web Notice That Each Water Molecule Can Potentially Form Four Hydrogen Bonds With Surrounding Water Molecules:
Web a single hydrogen atom can participate in two hydrogen bonds. Web the number refers to the number of bonds each of the element makes: Web so far, we’ve drawn this water molecule with one hydrogen bond. Thus one hydrogen atom will only bond once.
Such Molecules Will Always Have Higher.
Another hydrogen bond can be formed using the other lone pair on the oxygen atom. Covalent bonds require pairs of electrons and hydrogen can only have two electrons bound in one. Web methane, ( ch 4, is a single carbon atom covalently bonded to four hydrogen atoms. Web since hydrogen has only one valence electron, it will only bond once.
It Can Exist, For Instance, In Complex.
Each selenium atom can form two bonds, one with each hydrogen (2 hydrogen atoms total). Two with the hydrogen atoms and two with the with. Web apart from some group 13 weirdness, hydrogen can only make one bond.