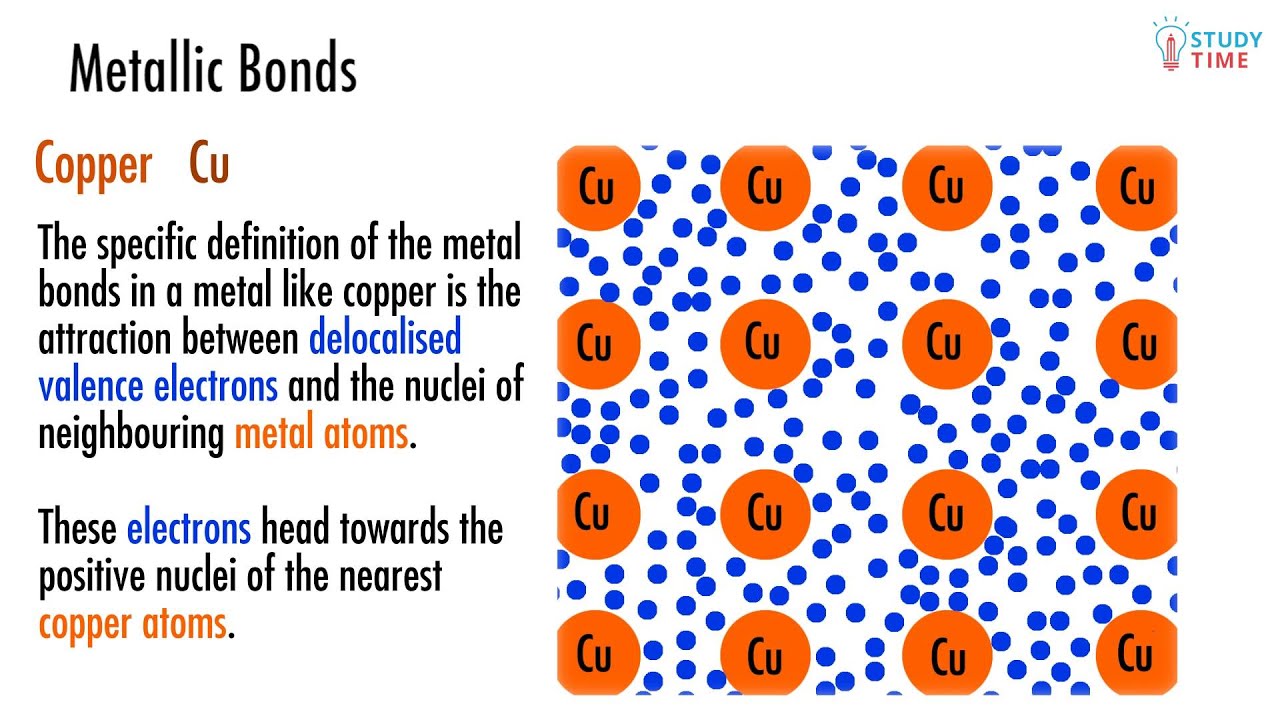
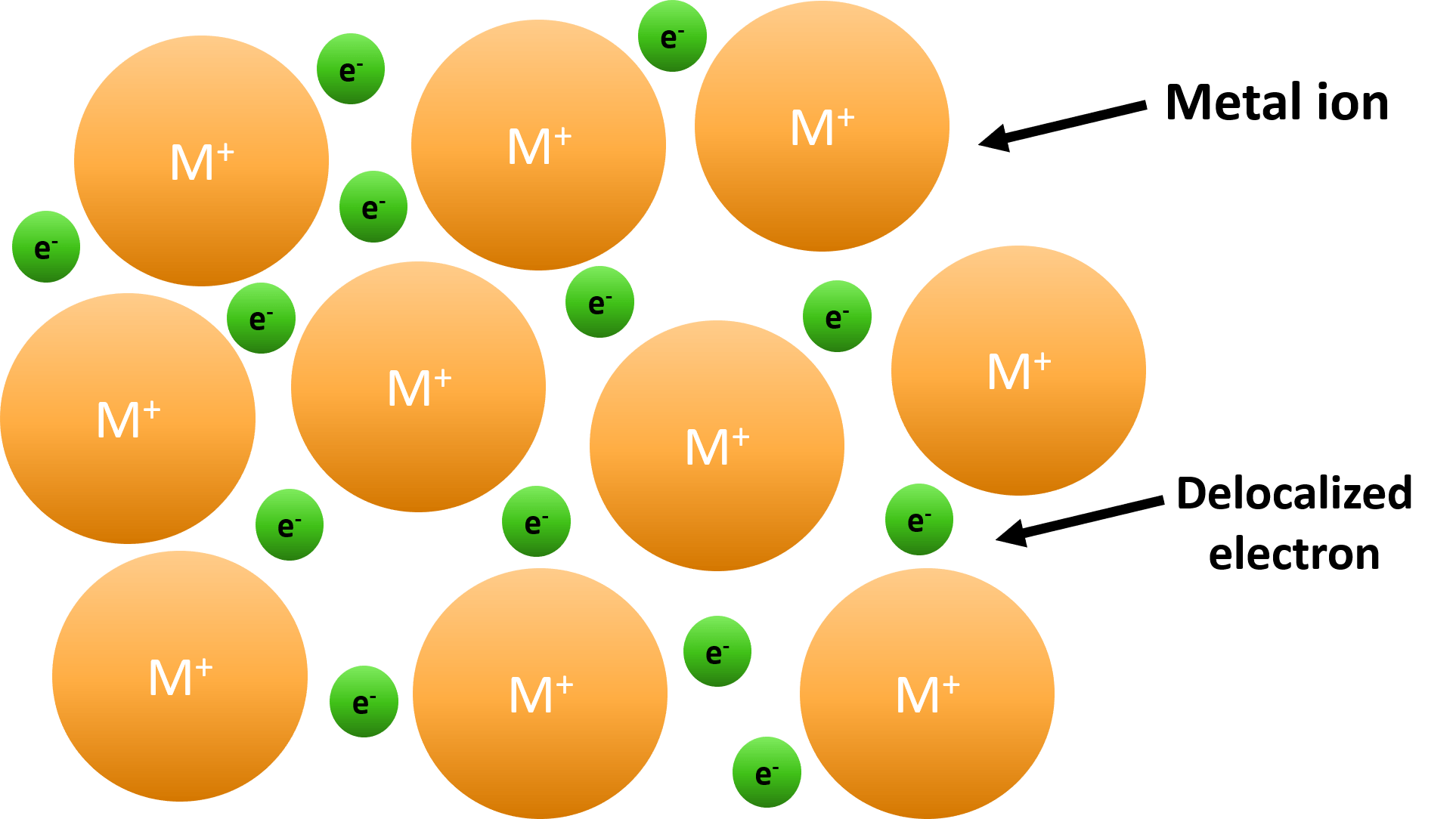
Which Atom Is Most Likely To Form A Metallic Bond
Which Atom Is Most Likely To Form A Metallic Bond - Its bonds are formed by large differences in electronegativity. Web answer:aluminium (al) atom is most likely to form a metallic bond.explanation:a kind of chemical bonding which generates between conduction electrons and positively Web metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized. Web when one atom takes an electron away from another and the resulting positive and negative ions are attracted to each other, those atoms have formed an ionic bond. A metallic bond is a type of chemical bond similar to a covalent bond. Aluminum (al) helium (he) phosphorus (p) carbon (c) aluminum (al) is most likely to form a metallic bond. Ionic bonding results from the electrostatic attraction of oppositely. Web a metallic bond is the way that metal atoms are kept together within a metal material. A kind of chemical bonding which generates between conduction electrons. It has only metallic bonds between the atoms.
Web a metallic bond is the way that metal atoms are kept together within a metal material. Web metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized. It has only covalent bonds between the atoms. Aluminium (al) atom is most likely to form a metallic bond. Metallic bonds are the kind of bonds that forms between metallic atoms. Web for example, covalently bonded gallium atoms tend to form crystal structures that are held together via metallic bonds. A metallic bond is a type of chemical bond similar to a covalent bond. Because metals are solid, their atoms are tightly packed in a regular. Aluminum (al) helium (he) phosphorus (p) carbon (c) aluminum (al) is most likely to form a metallic bond. A kind of chemical bonding which generates between conduction electrons.
Define electronegativity and assess the polarity of covalent bonds. Its bonds are formed by large differences in electronegativity. Aluminum (al) helium (he) phosphorus (p) carbon (c) aluminum (al) is most likely to form a metallic bond. A metallic bond is a type of chemical bond similar to a covalent bond. Aluminium (al) atom is most likely to form a metallic bond. Web which atom is most likely to form a metallic bond? Web this bonding occurs primarily between nonmetals; Which is most likely the result of millions of metal atoms crowding together so that molecular orbitals become combined? Web mostly, in the periodic table, left elements form metallic bonds, for example, zinc and copper. Web describe the formation of covalent bonds.
Chemistry B.Sc Level How many types of chemical bond
It has only covalent bonds between the atoms. Aluminum (al) helium (he) phosphorus (p) carbon (c) aluminum (al) is most likely to form a metallic bond. Web mostly, in the periodic table, left elements form metallic bonds, for example, zinc and copper. However, it can also be observed between nonmetals and metals. Web aluminum atom is most likely to form.
Bonds Between Atoms Sciencetastic!
Web mostly, in the periodic table, left elements form metallic bonds, for example, zinc and copper. Define electronegativity and assess the polarity of covalent bonds. However, it can also be observed between nonmetals and metals. Web metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron.
What Is Metallic Bonding Metallic Bonding and Structure YouTube
Web mostly, in the periodic table, left elements form metallic bonds, for example, zinc and copper. Web aluminum atom is most likely to form a metallic bond. Aluminum (al) helium (he) phosphorus (p) carbon (c) aluminum (al) is most likely to form a metallic bond. Web when one atom takes an electron away from another and the resulting positive and.
A carbon atom is most likely to form what kind of bond(s) with other
Its bonds are formed by large differences in electronegativity. Ionic bonding results from the electrostatic attraction of oppositely. A metallic bond is a type of chemical bond similar to a covalent bond. It has only covalent bonds between the atoms. A kind of chemical bonding which generates between conduction electrons.
Metallic Bonding Meaning, Properties, Factors Embibe
Web this bonding occurs primarily between nonmetals; Web mostly, in the periodic table, left elements form metallic bonds, for example, zinc and copper. Aluminum (al) helium (he) phosphorus (p) carbon (c) aluminum (al) is most likely to form a metallic bond. Web which atom is most likely to form a metallic bond? Ionic bonding results from the electrostatic attraction of.
Metallic Substances (8/12) Atomic Structure NCEA Level 2 Chemistry
Its bonds are formed by large differences in electronegativity. Web for example, covalently bonded gallium atoms tend to form crystal structures that are held together via metallic bonds. It has only metallic bonds between the atoms. It has only covalent bonds between the atoms. Web metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force.
Why Do Most Atoms Form Chemical Bonds? Sciencing
Web aluminum atom is most likely to form a metallic bond. The loss of metallic properties b. Web which atom is most likely to form a metallic bond? A kind of chemical bonding which generates between conduction electrons. The mercurous ion also exhibits metallic and covalent.
savvychemist Periodicity (2) Melting and boiling points of the
Web metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized. Web a metallic bond is the way that metal atoms are kept together within a metal material. It has only covalent bonds between the atoms. However, it can also be observed between.
1.6 Periodic Variations in Element Properties Chemistry for
Metallic bonds are the kind of bonds that forms between metallic atoms. Define electronegativity and assess the polarity of covalent bonds. Which is most likely the result of millions of metal atoms crowding together so that molecular orbitals become combined? Because metals are solid, their atoms are tightly packed in a regular. The loss of metallic properties b.
Metallic Bond — Formation & Compounds Expii
Aluminum (al) helium (he) phosphorus (p) carbon (c) a lead is malleable, so it can be pounded into flat sheets. Its bonds are formed by large differences in electronegativity. Because metals are solid, their atoms are tightly packed in a regular. Web for example, covalently bonded gallium atoms tend to form crystal structures that are held together via metallic bonds..
It Has Only Metallic Bonds Between The Atoms.
Metallic bonds are the kind of bonds that forms between metallic atoms. A metallic bond is a type of chemical bond similar to a covalent bond. Web mostly, in the periodic table, left elements form metallic bonds, for example, zinc and copper. Web answer:aluminium (al) atom is most likely to form a metallic bond.explanation:a kind of chemical bonding which generates between conduction electrons and positively
Aluminum (Al) Helium (He) Phosphorus (P) Carbon (C) Aluminum (Al) Is Most Likely To Form A Metallic Bond.
Web which atom is most likely to form a metallic bond get the answers you need, now! Ionic bonding results from the electrostatic attraction of oppositely. It has only covalent bonds between the atoms. Web when one atom takes an electron away from another and the resulting positive and negative ions are attracted to each other, those atoms have formed an ionic bond.
Web Which Atom Is Most Likely To Form A Metallic Bond?
Web for example, covalently bonded gallium atoms tend to form crystal structures that are held together via metallic bonds. Aluminum (al) helium (he) phosphorus (p) carbon (c) a lead is malleable, so it can be pounded into flat sheets. Which is most likely the result of millions of metal atoms crowding together so that molecular orbitals become combined? Web a metallic bond is the way that metal atoms are kept together within a metal material.
Because Metals Are Solid, Their Atoms Are Tightly Packed In A Regular.
Web this bonding occurs primarily between nonmetals; If atoms have similar electronegativities (the. Its bonds are formed by large differences in electronegativity. Web metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons (in the form of an electron cloud of delocalized.

:max_bytes(150000):strip_icc()/artwork-of-a-graphene-sheet-685024423-5c49e1cdc9e77c0001b8e3e9.jpg)